featured
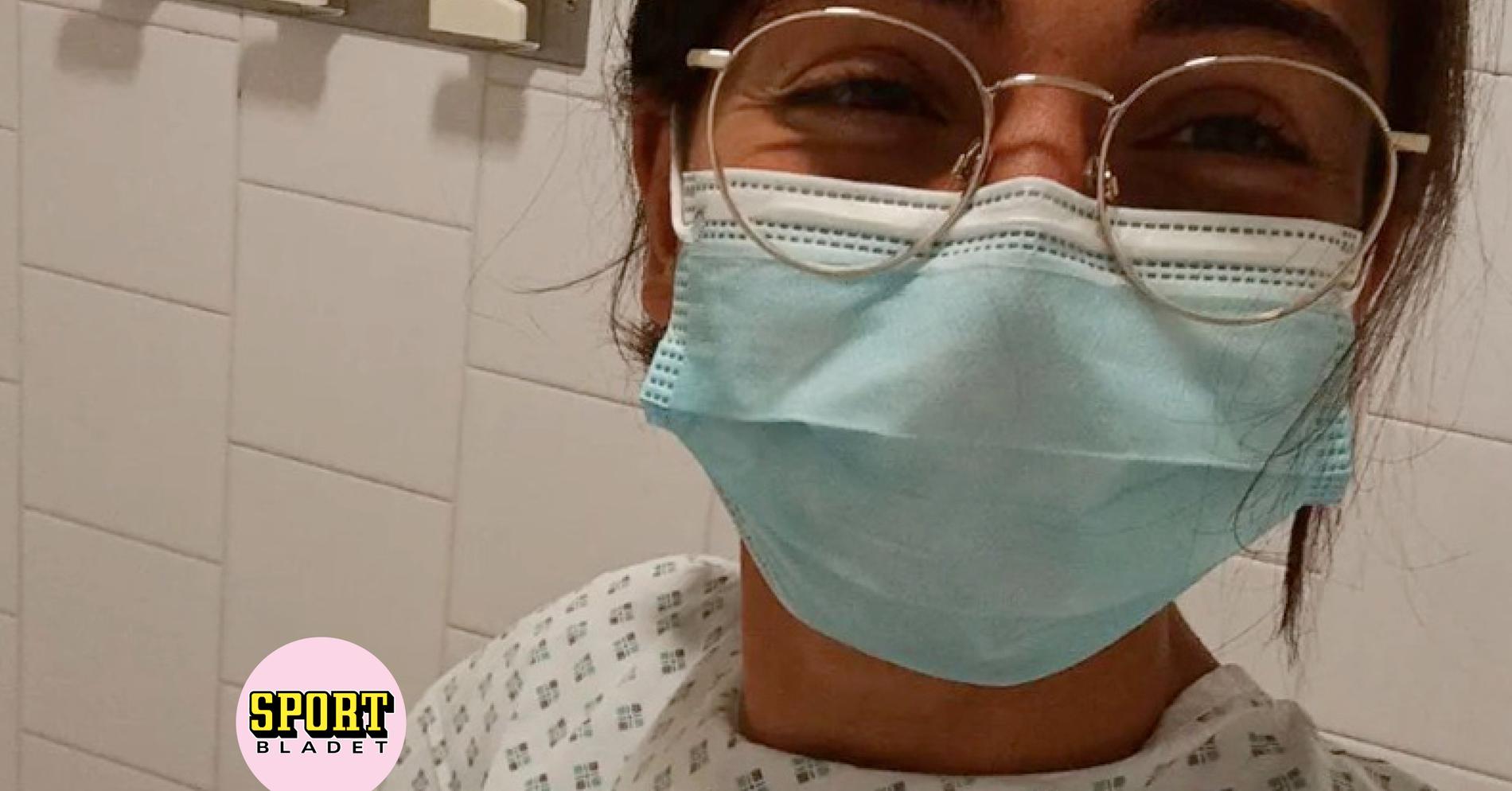
Sara Benfares banned for five years – blamed on cancer
Published 2024-04-22 17.09 share-arrowDela unsaveSpara Sara Benfares, 22, ended up in a storm after a positive doping test. She was accused of lying about cancer. Now the German distance runner has been suspended for five …

Sara Benfares banned for five years – blamed on cancer
Published 2024-04-22 17.09 share-arrowDela unsaveSpara Sara Benfares, 22, ended up in a storm after a positive doping test. … Read more

The US restricts trips of its employees to Chiapas due to insecurity
Mexico City. Due to increased violence and security concerns in Chiapas, the United States government this Friday restricted … Read more

Acapulco, halfway through its recovery after Otis
Acapulco Guerrero. More than 10,000 members of the National Guard are stationed in the port of Acapulco to … Read more

Colombia suspends state work for a day to save water
Bogota. The Colombian government ordered the suspension of the Friday work day for public officials in order to … Read more
Culture and tradition dance at the “Ximopano No Kali” Festival
Tepetlaoxtoc, Mex. Dance, tradition, history and culture came together at the third “Ximopano No Kali” Dance Festival held … Read more



