Current newsEnglish – Of course this technique has been a dream of mankind for centuries. Now scientists say they have found a way to reverse the aging process in human skin.
Reported from Dailymail.co.ukon April 24, researchers at Cambridge revealed that they had reprogrammed the skin cells of people aged 38 and 53 to make them ‘younger’ than they were at 30.
–
This method delays the aging clock even further than previous reprogramming methods without damaging cells.
The researchers say they were even able to restore some of the function that had been lost in the older cells.
While the research is still in its early stages, the findings could eventually revolutionize regenerative medicine, especially if it can be replicated in other cell types and other tissues in the body, the researchers claim.
Related news :
Chemical Pollution of Household Products Lowers Male Sperm Production
–
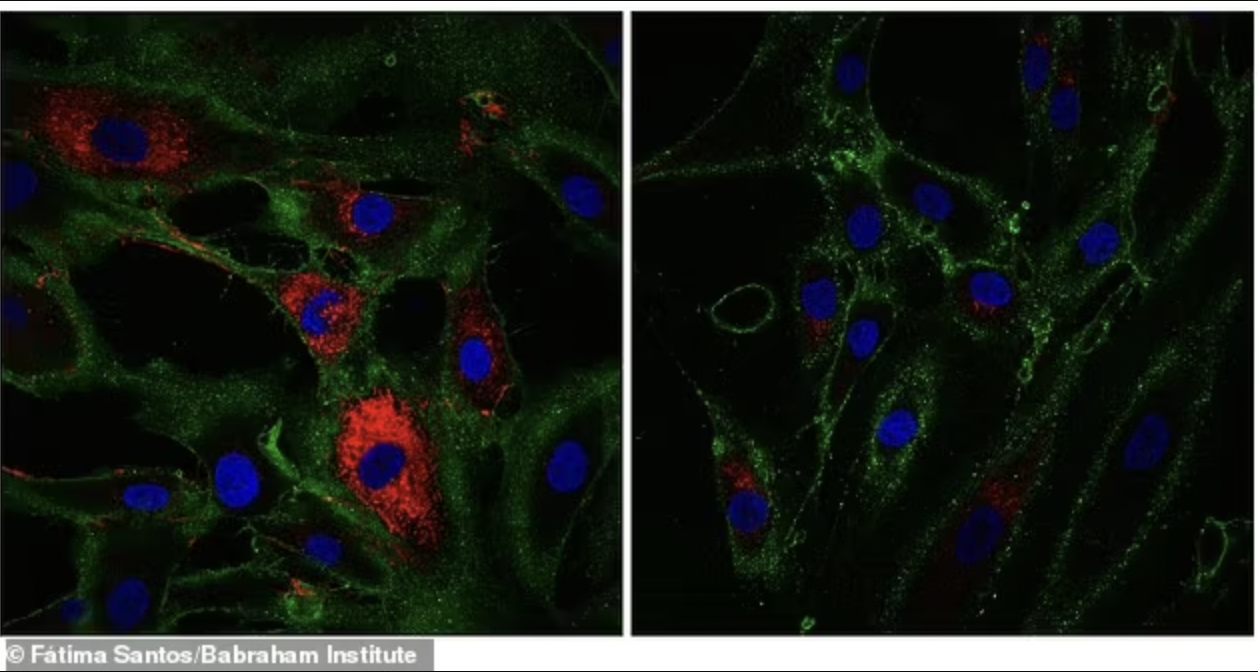
In the experiment, aging cells became more like skin cells called fibroblasts that produce collagen, the protein that holds the body together and keeps it strong.
The number of fibroblasts in human skin decreases progressively with age. These cells also become wrinkled with age.
The new findings could lead to targeted approaches to treating aging, which could ‘revolutionize’ regenerative medicine, according to the researchers.
The new research was conducted at the Babraham Institute, a life sciences research institute in Cambridge, and published in the journal eLife.
“Our results represent a major step forward in our understanding of cell reprogramming,” said Dr Diljeet Gill at the Babraham Institute.
“We have proven that cells can be rejuvenated without losing their function and that rejuvenation is seen to restore some function to old cells.”
“The fact that we also see reversal of aging indicators in disease-associated genes holds great promise for the future of this work.”
As they age, their cells’ ability to function declines and their genomic DNA blueprints accumulate signs of aging.
Related news :
Internet Helps Parents Fight Depression During Lockdown
–
Regenerative biology aims to repair or replace cells, including old ones.
One of the most important tools in regenerative biology is our ability to create ‘induced’ stem cells.
However, this process essentially removes cells from their function and gives them the potential to become any type of cell.
His early work stemmed from work at the Roslin Institute in Edinburgh in the 1990s to take milk cells taken from six-year-old sheep into embryos.
This project famously led to the creation of the Dolly sheep, the first mammal to be cloned from an adult somatic cell.
Dolly’s creation showed that genes in the nucleus of an adult cell can still return to an embryonic totipotent state, meaning cells can divide to produce all the different cells in animals.

This paved the way for Nobel Prize-winning scientist Dr Shinya Yamanaka, who in 2007, was the first scientist to convert normal cells, which have a special function, into stem cells that have the special ability to develop into any cell type.
Related news :
It turns out that consuming mushrooms once a day can reduce the risk of cancer
–
The method, called IPS, takes about 50 days using four key molecules called Yamanaka transcription factors, Oct4, Sox2, Klf4 and cMyc.
Babraham Institute’s new method, called ‘transient reprogramming of the maturation phase’, exposed cells to Yamanaka factor in just 13 days instead of 50.
At this point, the cells do not turn into embryonic stem cells, but become “rejuvenated” as if they were 30 years younger.
The partially reprogrammed cells were given time to grow under normal conditions, to observe whether their specific skin cell function returned.
Genomic analysis showed that the cells had regained the characteristic markers of skin cells (fibroblasts), and this was confirmed by observing collagen production in the reprogrammed cells.
To show that the cells have been rejuvenated, the researchers looked for changes in signs of aging.

The researchers looked at several measures of cellular age. The first is the epigenetic clock, where chemical tags present throughout the genome indicate age.
The second is the transcriptome, all the gene readings produced by the cell.
With these two measures, the reprogrammed cells matched the profile of cells that were 30 years younger than the reference data set, the team said.
This technique could not immediately be translated into clinical settings because IPS was found to increase the risk of cancer.
Currently the next research step is to understand the exact mechanism that allows this partial reprogramming, but it could eventually be used for cellular therapy in situations where cell age makes a difference, such as in skin healing for burns. .
The potential application of this technique relies on cells not only looking younger, but also functioning like young cells.
Fibroblasts produce collagen, a molecule found in bones, skin tendons and ligaments, helping to provide tissue structure and heal wounds.
The rejuvenated fibroblasts produced more collagen proteins than control cells that did not undergo the reprogramming process, Babraham Institute researchers found.
Fibroblasts also moved to areas that needed repair, so the researchers tested partially rejuvenated cells by making artificial cuts in the cell layers on a plate.
They found that treated fibroblasts moved into crevices faster than older cells, a promising sign that could one day be used to make cells that are better at healing wounds.
The researchers also wanted to see if the new method would work on other tissues such as muscle, liver and blood cells.
In the future, this research may also open up other therapeutic possibilities; the researchers observed that their method also had effects on other genes associated with disease and age-related symptoms.
The APBA2 gene, which is associated with Alzheimer’s disease, and the MAF gene, which plays a role in cataract development, both exhibit changes to younger transcriptional levels.

The mechanics behind the success of temporary reprogramming are not yet fully understood, and are the next piece of the puzzle to explore.

–
–