WITH
–
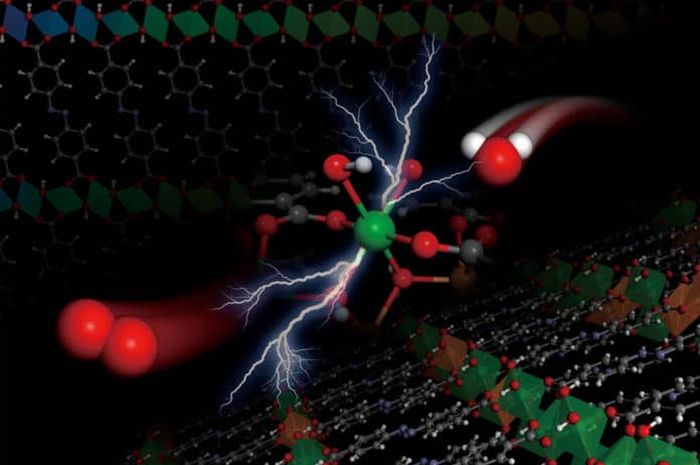
The illustration depicts the electrochemical reaction, the splitting of a water molecule (on the left, with an oxygen atom in red, and two hydrogen atoms in white) into an oxygen molecule (on the right), occurring within the structure of the metal hydroxide team’s organic framework, depicted as a lattice at the top and bottom. lower.
–
Nationalgeographic.co.id – Electrochemical reaction that breaks water molecules to produce oxygen is at the heart of the various approaches that aim to produce alternative fuels useful in the transportation sector. However, this reaction must be facilitated by catalystand current versions require the use of rare and expensive elements such as iridium, which limits the production potential of these fuels.
Now, researchers at MIT and elsewhere have developed an entirely new type of catalyst material, called metal hydroxide-organic framework (MHOF), which is made of cheap and abundant components. This group of materials allows engineers to precisely match the structure and composition of the catalyst to the needs of a particular chemical process, and can then match or exceed the performance of more expensive conventional catalysts.
The findings have been published in the journal Nature Materials on February 24, 2022 entitled “Tunable metal hydroxide–organic frameworks for catalysing oxygen evolution”, a paper co-authored by MIT postdoc Shuai Yuan, graduate student Jiayu Peng, Professor Yang Shao-Horn, Professor Yuriy Román-Leshkov, and nine others.
The oxygen evolution reaction is one of the common reactions for the electrochemical production of fuels, chemicals, and materials. These processes include the generation of hydrogen as a by-product of oxygen evolution, which can be used directly as a fuel or undergo chemical reactions to produce other transportation fuels; manufacture of ammonia, for use as fertilizer or chemical feedstock; and carbon dioxide reduction to control emissions.
Also Read: Why is the 2021 Nobel Prize in Chemistry’s Organic Catalyst So Special?
But without help, “This reaction is sluggish,” said Shao-Horn. “For reactions with slow kinetics, you have to sacrifice voltage or energy to increase the rate of the reaction.” Because of the extra energy input required, “The overall efficiency is low. So that’s why people use catalysts,” he says, because these materials naturally promote reactions by lowering the energy input.
“Other teams have explored the use of metal hydroxides, such as nickel-iron hydroxide. But such materials are difficult to adapt to specific application requirements. Now, however, the reason our work is quite interesting and quite relevant is that we have found a way to adjust the properties by uniquely fabricating the nanostructures of this metal hydroxide,” said Professor Yuriy Román-Leshkov, as reported Tech Explorist.

petrmalinak, Shutterstock
–
This process includes the generation of hydrogen as a by-product of oxygen evolution, which can be used directly as a fuel or undergo chemical reactions to produce transportation fuels.
–
The team borrowed from research that had been done on a class of related compounds known as metal-organic frameworks (MOFs), which is a kind of crystalline structure made of metal oxide nodes linked together with organic linking molecules. By replacing the metal oxides in the material with specific metal hydroxides, the team found, it became possible to fabricate a precisely adjustable material that also has the stability needed to be potentially useful as a catalyst.
“You place these organic linking chains next to each other, and they actually lead to the formation of metal hydroxide sheets which are interconnected by these organic links, which then stack, and have higher stability,” Román-Leshkov said. This has many benefits, he said, by enabling precise control over the nanostructured patterns, enabling precise control of the electronic properties of metals, and also providing greater stability, allowing them to stand up to long periods of use.
PROMOTED CONTENT
Featured Videos
–