Input 2021.02.02 10:14 | Revision 2021.02.02 10:18
mRNA method Pfizer Modena, 95% prevention
Traditional Nova Vax, safe at room temperature distribution
Astra introduced this month, controversy over vaccination for the elderly–
–
According to the Ministry of Food and Drug Safety and the foreign press on the 2nd, the most effective vaccine is the Pfizer and Moderna vaccine, a family of’messenger ribonucleic acid (mRNA)’ vaccines. In terms of safety, NovaVax vaccine, an existing’antigen synthesis’ vaccine family, is evaluated to be the highest.
Pfizer and Moderna, respectively, announced that their vaccines showed 95% and 94.1% preventive effects in clinical practice. In fact, a local media reported on the 28th of last month (local time) that Israel, which recently started vaccinating Pfizer, showed a 92% prevention effect in an evaluation of 163,000 vaccinators.
The downside is that storage and distribution are difficult. Unlike conventional vaccine components, mRNA is easily deteriorated at room temperature, so a cold chain (low temperature distribution system) is required. Pfizer and Moder require a cryogenic environment of -70 degrees Celsius and 20 degrees Celsius, respectively.
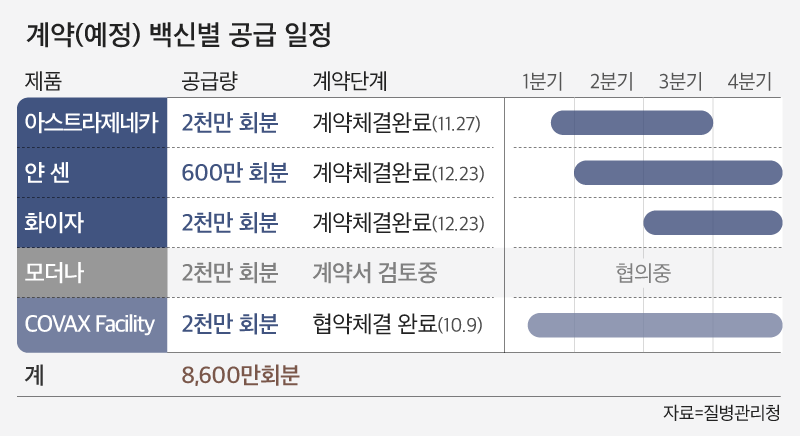
Korea, which is bringing about 60,000 doses (117,000 doses) of Pfizer vaccine through Cobax Facility this month, decided to build 250 vaccination centers equipped with freezers nationwide. The day before, the National Medical Center Central Vaccination Center appeared as the No. 1 vaccination center.
The disadvantage is that both vaccines are currently in short supply than other vaccines. Recently, some countries, such as France and Spain, temporarily stopped vaccination due to a lack of supply.
Korea will introduce a total of 10 million people from the third quarter through a direct contract with Pfizer. Modena vaccine plans to bring in 20 million people from the second quarter.
–
–
Unlike Pfizer, modder or vaccine, it is easy to distribute and store. It can be stored at 2 to 8 degrees Celsius, so it can be inoculated in general medical institutions without a cold chain including a cryogenic freezer and an inoculation center.
However, it is pointed out that the data on efficacy and safety are insufficient for the elderly, who are eligible for priority vaccination. Pfizer and Moderna exceeded 20% of the subjects who participated in the vaccine clinical trial, but AstraZeneca only reached 10%, and further verification is needed to determine whether vaccination for the elderly is possible. For this reason, some European countries, such as Germany and France, recently made recommendations to limit vaccination for the elderly.
The previous day, the Ministry of Food and Drug Safety said, “At a verification advisory meeting, a number of experts gave an opinion that the elderly could not be excluded from the vaccination target.” Immediately after the announcement of the Ministry of Food and Drug Safety, the president of the Korean Medical Association said, “Seniors over 65 years of age should not be vaccinated (because safety verification has not been completed),” and indicated the possibility of conflict between health authorities and the medical community over this issue in the future. . The Ministry of Food and Drug Safety will hold the second advisory group (Central Pharmacy Review Committee) meeting on the 4th to discuss this issue again.
The preventive effect was 62%. It is lower than Pfizer and Moder or Vaccine, but the Ministry of Food and Drug Safety evaluated that it satisfies ‘50% or more’, which is the evaluation standard of the World Health Organization (WHO). It is an evaluation that the safety standards have also been met.
–
–
NovaVax vaccine is made with the same synthetic antigen method as many conventional vaccines, and it is evaluated that it is safe compared to the first attempted mRNA. The preventive effect was found to be 89.3%, but like Janssen, the preventive effect against South African mutations was only 49%. This vaccine has not yet been confirmed in Korea. The government has introduced 20 million people from the second quarter as early as possible and is promoting a plan to consign domestic production through SK Bioscience.
–
–
– .


