‘We won’, hurry to review the rights, the first round, the last day! Feb. 21
21 February 2021 | by Bangkok Business Online – 1,137 – The last day! Hurry to review the right “We won” Round 1 within 21 February 2020 prepare to receive money into “Pao Tang” 11 Mar Remind each other again For registrants, “We won” and found unauthorized messages. Able to apply for rights review through … Read more



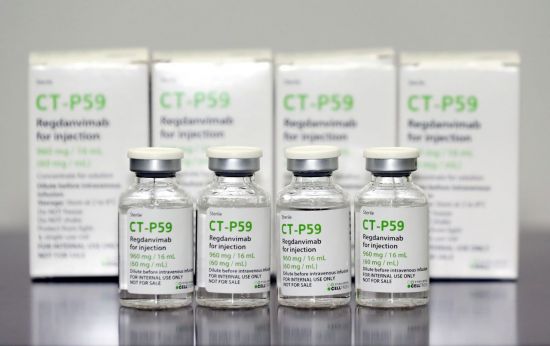
![[비즈헬스] Celltrion opens the era of’domestic Corona 19 treatment’… Conditional approval of antibody treatment’Rekirona’ [비즈헬스] Celltrion opens the era of’domestic Corona 19 treatment’… Conditional approval of antibody treatment’Rekirona’](https://cdn.bizwnews.com/news/thumbnail/202102/23098_23415_3515_v150.jpg)

![[특징주] Shinpoong Pharmaceutical, Piramax Corona 19 Global Clinical Phase 3 News’Strong’ [특징주] Shinpoong Pharmaceutical, Piramax Corona 19 Global Clinical Phase 3 News’Strong’](https://www.etoday.co.kr/images/etoday_og.jpg)

