Lenacapavir (GS-6207 or LEN) is the first HIV-1 capsid inhibitor acting at several stages of the viral replication cycle, developed in long acting. A single injection of LEN maintains optimal levels for 26 weeks and therefore allows it to be administered every 6 months.
–
According to Segal-Maurer S et al., Abstr. 127, and VanderVeen L et al., Abstr. 128, updated.
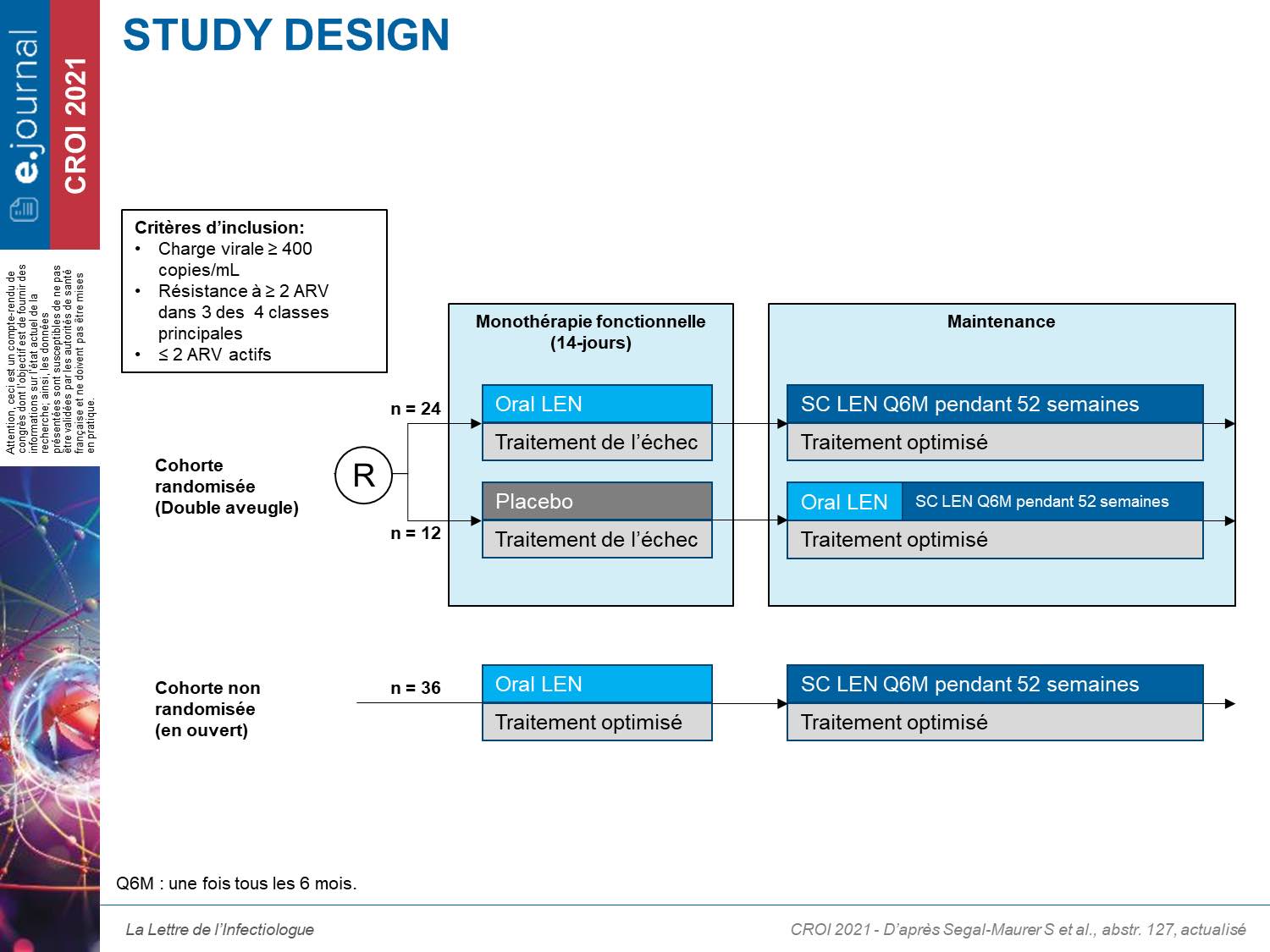
This is a phase II / III, randomized, double-blind study comparing LEN as monotherapy for 14 days versus placebo (600 mg on D1 and D2 and 300 mg on D8 orally), in patients with largely pretreated patients in virological failure with documented resistance to at least 2 ARVs in at least 3 therapeutic classes. The patients were then included in a maintenance treatment with injectable LEN (927 mg) every 6 months associated with an optimized treatment after oral treatment in the placebo group (study design below).
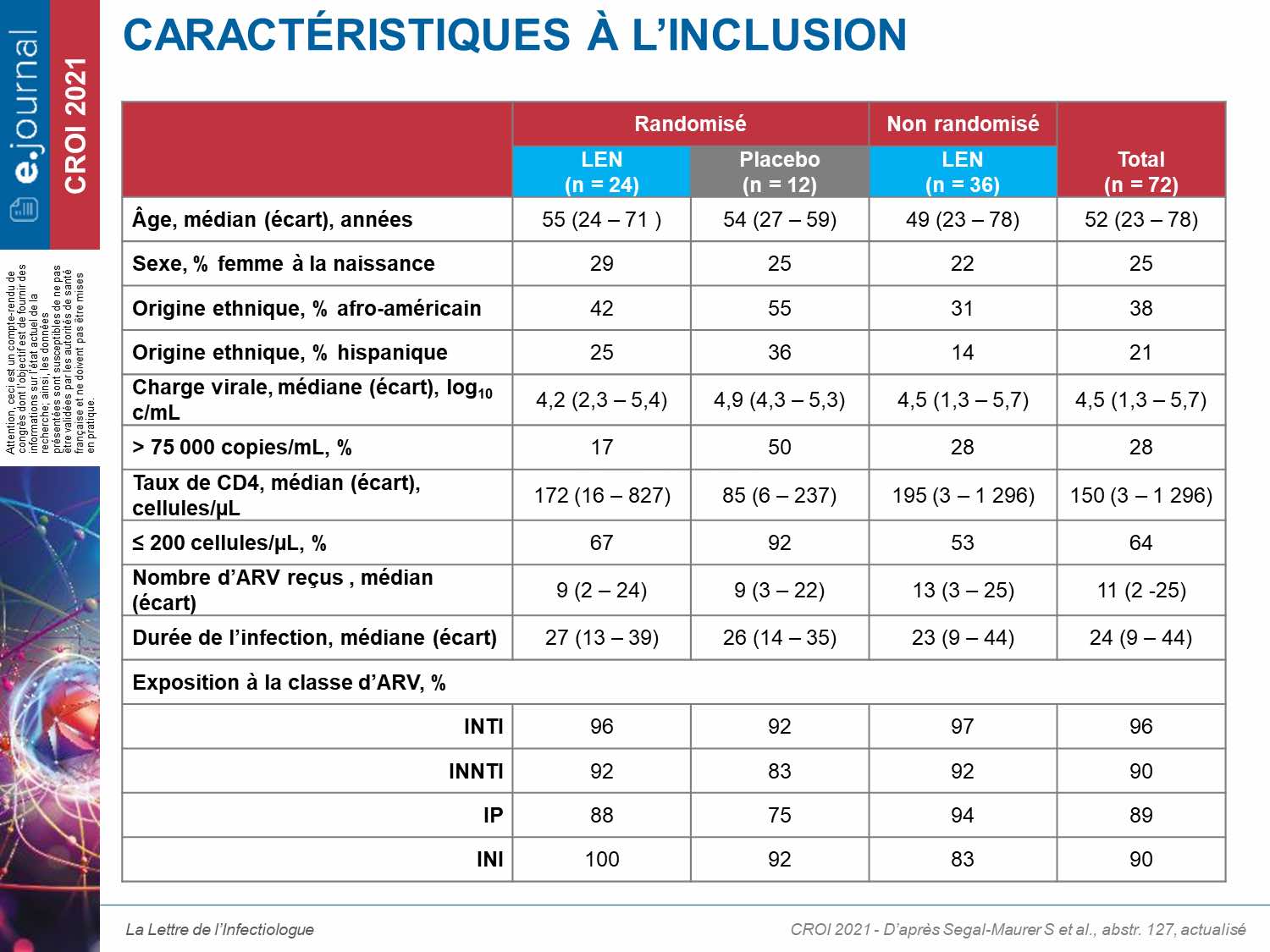
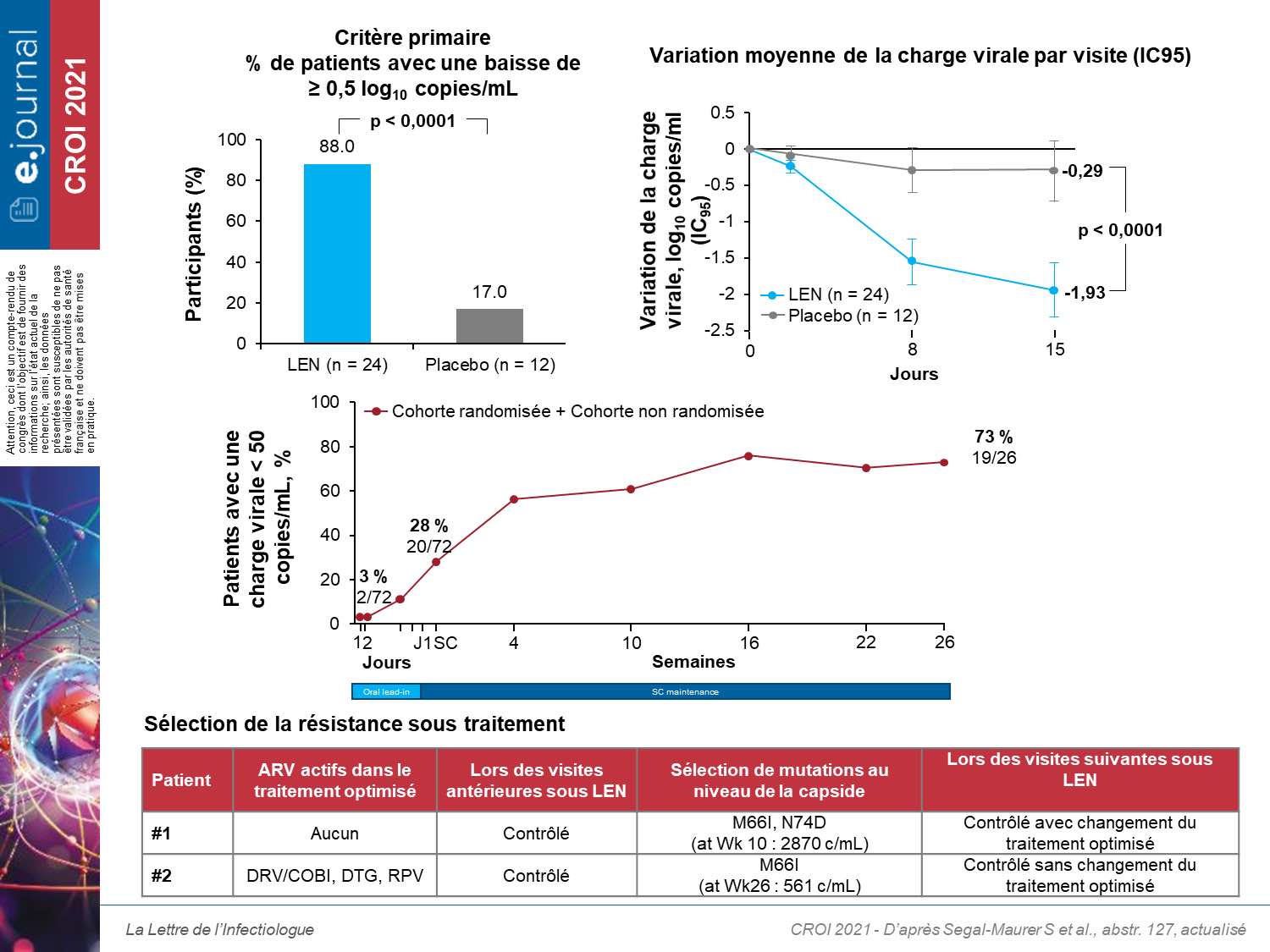
The primary efficacy endpoint was the proportion of participants with a decrease in CV of at least 0.5 log10 cp / mL on D15. 36 patients were randomized with a mean CV level of 4.27 log10cp / mL. At D15, 88% on LEN (21/24) had a decrease of at least 0.5 log10 cp / mL compared to 17% on placebo (2/12) (difference of 71%, p <0001). The variation of CV (log10 cp / mL) was significantly different from −1.93 versus −0.29. 24 weeks after the first injection of LEN associated with the optimized treatment, 73% of patients had a CV <50 cp / mL at W24. Out of all 72 largely pretreated patients from the 2 cohorts, 2 patients presented a selection of resistance to LEN with the selection of the M66I mutation isolated for one patient and associated with N74D for the second. There were no serious treatment-related adverse effects, discontinuation or death. The most common side effects were injection site reactions (46%).
The mutations identified under selection pressure in vitro are 7 (L56I, M66I, Q67H, K70N, N74D, N74S and T107N) conferring a reduced sensitivity to LEN, in particular with M66I leading to a resistance of more than 2,700 times and reduced replicative capacity. In this in vitro study, LEN was effective on strains resistant to PIs and on all subtypes with levels of IC50 similar.
These data demonstrate the interest of the development of lenacapavir in the treatment and prevention of HIV infection.
This article was originally published in the e-journal of “The Infectiologist’s Letter” covering the conference, we reproduce it here with their kind permission.
–