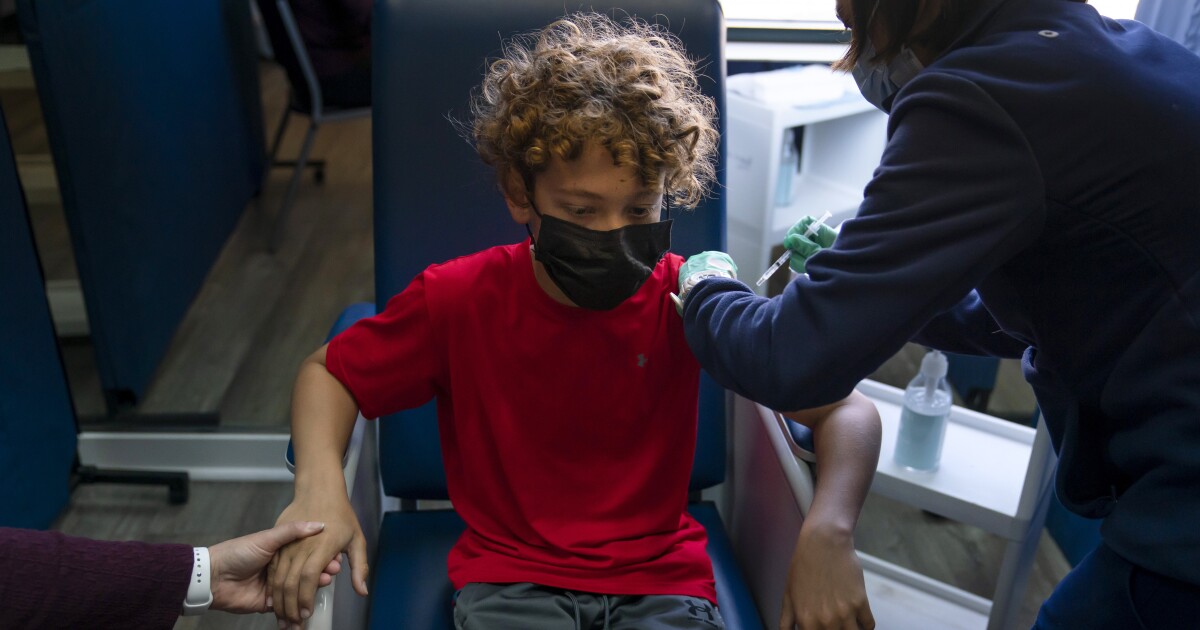
If you are the parent of a young child, a school employee, or simply someone who pulls on their mask a little tighter when they see an approaching group of children, you are probably asking yourself this question with increasing urgency:
How soon can younger Americans get the vaccine safely?
Be prepared to hear that you must be patient and also to hear the often repeated mantra of pediatric medical professionals: Children are not little adults.
In other words, don’t assume that because COVID-19 vaccines have been shown to protect adults and teens fairly safely, the same vaccines at the same doses will work just as well for younger children.
Children’s bodies – their organs, muscles, and bones, and their metabolic and immune systems – function differently from those of adults. This means that they may respond very differently to older adult medications.
So before COVID-19 vaccines can be approved for the 28 million American children – between the ages of 5 to 11 – and the 20 million who are even younger, regulators need strong evidence that vaccines are safe and that reduce the risk of contracting the disease.
That evidence will have to come from clinical trials that will be analyzed by experts from the Food and Drug Administration (FDA). It is usually a rigorous and deliberate process, often taking years.
But amid a pandemic accelerated at full speed by the appearance of the Delta variant, “demanding and deliberate” begins to sound more like “slow and bureaucratic.”
Between late June and mid-August, when the Delta variant consolidated its grip on the nation, the rate of hospitalization of children for COVID-19 increased almost fivefold. The nation’s pediatricians were growing impatient.
“The Delta variant has created a new and pressing risk for children and adolescents across the country,” the American Academy of Pediatrics wrote in a letter to the FDA on Aug. 5. Doctors urged the agency to “carefully consider the impact of its regulatory decisions and their impact on further delays regarding the availability of vaccines for this age group.”
The FDA is well aware of the pressure. It responded on Friday with the release of an unusual public statement recognizing the urgency of its mission and promising to review clinical trial data “as quickly as possible, probably in a matter of weeks rather than months.”
Here’s a closer look at where we are going from where we are right now.
Aren’t some COVID-19 vaccines already available to American children?
Yes. The one made by Pfizer and BioNTech has received full approval for individuals 16 years of age and older and has been licensed for emergency use in children 12-15 years of age.
Is this so?
Up to here, yes. Pfizer said it expects to have the first results of its trial in younger children to submit to the FDA later this month or earlier. October. Once that data is submitted, the FDA can begin to evaluate it.
A second vaccine manufactured by Moderna obtained provisional approval for adolescents ages 12 to 18 in Canada, Europe and Japan. The company has applied for FDA approval for emergency use in children in the US from the age of 12, and said the results of its clinical trials were “consistent with 100% vaccine efficacy. “An advanced stage test in 4,000 minors – from 5 to 11 years old – has just completed the registration.
The Johnson & Johnson vaccine is still being tested in teens ages 12 to 17.
What is slowing things down?
Concerned about the emergence of a couple of rare side effects, the FDA, in mid-summer, spoke again with vaccine manufacturers and told them to add more children to their trials. Regulators also proposed that they track trials longer than they had initially planned. That would give safety experts a better chance of detecting unusual side effects, as well as very late reactions to the vaccine.
Pfizer and Moderna responded by roughly doubling the size of their tests for younger children.
The sudden change alarmed Stanford pediatrician Dr. Yvonne Maldonado, who chairs the American Academy of Pediatrics Committee on Infectious Diseases and is working on one of Pfizer’s pediatric clinical trials.
“I would have extended the schedule by several months,” he said. And statistically, a trial with twice as many children probably still wouldn’t be enough to detect or stop a rare side effect. Also, since post-vaccine reactions are more likely to occur within two months of receiving the injection, lengthening follow-up is unlikely to add information, he said.
Why would regulators risk a delay?
The challenge of clinical trials is that ‘essentially, you’re trying to predict the safety of a vaccine in billions of children by looking at its safety in thousands of them,’ said Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia
It’s important to try to detect rare adverse effects, said Offit, who has advised both the FDA and the Centers for Disease Control and Prevention. Even if a terrible side effect occurred in just 1 in 1 million children, it could mean vaccine-related hospitalizations or the death of up to 120 children in the US alone.
But, during a terrible pandemic in which children are being hospitalized at an increasing rate, it is costly to extend trials in the hope of picking up weak safety signals. “It is frustrating that the minors have gone back to school and are not vaccinated,” said Offit.
Is the FDA looking for something specific?
Yes. Regulators are particularly interested in learning more about a condition called myocarditis, which is swelling or inflammation of the heart muscle.
In early June, just a month after the Pfizer vaccine became available to 16- and 17-year-olds, the CDC (Centers for Disease Control and Prevention) recorded a slight increase in myocarditis cases in newly vaccinated people.
This collateral effect has been seen mainly in boys and men under 30 years of age; symptoms were generally mild and disappeared within a few days, either alone or with over-the-counter medications. The long-term effects of a mild case of myocarditis are unclear.
At the same time, myocarditis is often seen in children and young adults who are hospitalized with COVID-19, so preventing the disease with a vaccine can be a net gain. Research is ongoing, and pediatricians aren’t sure that younger children are as prone to side effects as their older siblings.
After a detailed briefing in June, a CDC advisory panel recommended the vaccine for all eligible teens. But the panel cautioned that people who developed myocarditis after their first dose should consider delaying their second until they have recovered or the condition is better understood.
Is this so?
There is always the possibility of a completely unforeseen side effect, such as blood clots that emerged in a smaller and smaller number of younger women who received the J&J vaccine. Although the administration of that vaccine was briefly paused while the risk was investigated, its application was quickly resumed.
In the midst of a pandemic, detecting rare or unforeseen side effects is a task best left behind until after a vaccine is licensed and large numbers of diverse people begin contracting it, Maldonado said.
“This wait-and-see approach assumes you know what to expect,” Maldonado said. “We cannot afford to wait three to five years, whatever that may be, for the magical end point.”
As long as children are not vaccinated, they will continue to transmit the virus and keep the pandemic alive, he added.
Wouldn’t additional FDA statistics guarantee that vaccines are safe for children?
With vaccines, especially for children, medical ethicists demand a particularly high level of safety. After all, vaccines are given to healthy people who, if they are lucky, may never be exposed to the disease-causing pathogen.
Therefore, in the best of cases, tolerance to risk vaccines is very low. But now, regulators face a dilemma.
Public distrust continues to stifle acceptance of the vaccine and drive COVID-19 hospitalizations. If the FDA intensifies its efforts to detect vaccine-related side effects, will it strengthen the public’s confidence in its oversight? Or will it erode faith in vaccines by pursuing problems that may prove harmless?
To read this note in English please click here
–