Searching for a child can be a rewarding path without too many difficulties, but at other times, it can be winding, distressing, frustrating and even costly from a financial point of view.
Thinking about those difficulties in parenting and how to solve them, it emerged Selectivity, a Cordoba startup that is developing a product aimed at universalizing fertility treatments. It was designed so that couples can use a fertilization kit at home, as a first option, at an affordable cost.
This company that has just started and that due to its added value has the support and accompaniment of the Ministry of Science and Technology of Córdoba and the Innovar y Emprender Agency, was established in February 2019, but began its journey some time before, with the work of one of its founders, who dedicated himself to the study of sperm selection.
In his analytical journey, the researcher observed that there are some underlying problems with current techniques: the best cells are not always separated, sometimes the DNA is damaged, fragmenting; then, when it comes to the insemination process, not always the best sperm are inside the corresponding cannula to be placed in the woman’s uterus. For this reason, a new selection method was designed that is registered under intellectual property.
The Selectivity team is composed of Herberto Repetto, biochemist; Leandro Reartes, industrial engineer and Jonathan Gubspun, bioengineer.
How will they achieve the universalization of treatments?
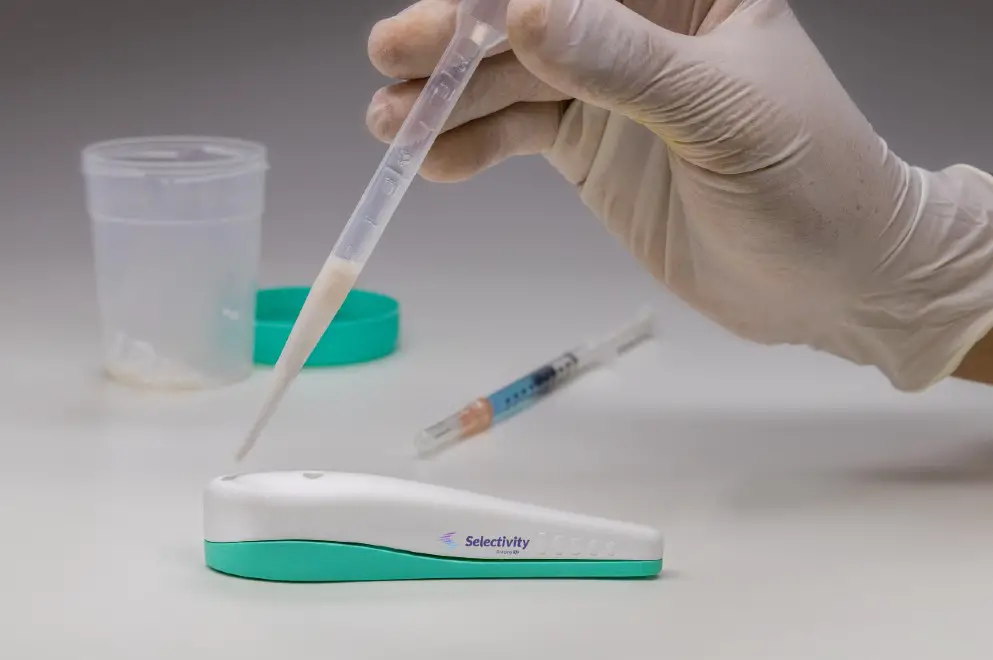
Selectivity is currently developing three products. The first is a device of sperm selection, which separates the most mobile spermatozoa and suitable for fertilization in a non-invasive way.
This has two important competitive advantages: una is that it does not require highly qualified personnel or any careful environment for its use. In this way, in any environment, such as the house, a sperm selection can be made. The second advantage is that as it is non-invasive, it does not contribute to DNA fragmentation, that is, it better conserves the genetic material of the cells, compared to traditional methods.
This product is undergoing regulatory approval by the National Administration of Medicines, Food and Medical Technology (ANMAT) and expects to have news approximately in August.
The second device, known as insemination assist, helps the user to identify the cervix and insert the insemination cannula into it. and at the same time it can transmit these images online, making it possible for untrained personnel to safely perform intrauterine insemination.
Using these two products, anyone can carry out a low complexity fertility treatment. Even so, they remain two devices that have a certain complexity in handling, using needles and cannulas. This is why Selectivity faces its greatest challenge in developing a third product: a home insemination system. It is a device that anyone can buy in a pharmacy or online and with which they can carry out intrauterine insemination treatment at home.
Home insemination
This third product, the home insemination system under development, it is with which Selectivity intends to universalize fertility treatments.
In general, a couple reaches a fertilization treatment after 12 months of trying to conceive naturally. This creates stress, fear, and anxiety; furthermore, they find that there are not specialized centers in all cities.
“With this device the amounts are significantly reduced. In this way, the aim is to give the possibility to those couples who have doubts about their fertility and that, when they wish, acquire a Selectivity device and practice insemination treatment at home.“, Assured the entrepreneurs.
“This device would be to be used in the first stage of fertility treatments. Just as a couple who doubts if they got pregnant can buy a pregnancy test, the idea is that those who are not able to get pregnant look for this fertilization kit as the first option. If she does not achieve pregnancy, she will have to seek a more complex treatment ”, they explained.
Actually, Selectivity began an investment search round in order to continue the development of this product and carry out clinical tests. Therefore, expected to hit the market in early 2023.
–