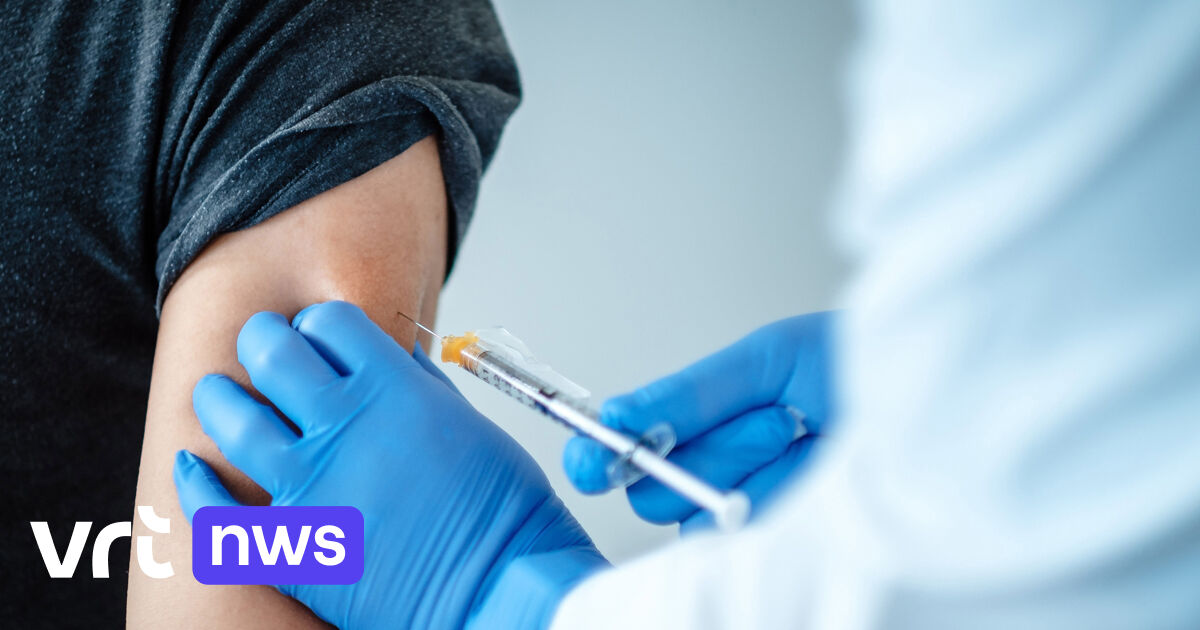
The UK is close to launching a COVID-19 vaccine campaign.
UK National Health Service employees, nursing home residents and their caregivers will begin receiving one of two doses of the vaccine, which was jointly developed by American pharmaceutical company Pfizer and German pharmaceutical company Bioendech.
 —
—–
The initiative comes nearly a week after the government’s medical regulatory agency allowed the vaccine to be used immediately, making Britain the first Western country ready to launch mass immunization. The approval comes just weeks after Pfizer said the vaccine had demonstrated efficacy of more than 90 percent following the latest comprehensive clinical trial.
The UK received 800,000 doses of Pfizer / Bioendech vaccine on Sunday, buying a total of 40 million doses. Vaccination is complicated by the fact that the vaccine must be stored in the refrigerator at a very low temperature, minus 70 degrees Celsius.
Queen Elizabeth II, 94, and her husband, Prince Philip, 99, will announce when they will be vaccinated, British media reported on Sunday.
In a separate development, the Serum Institute of India has applied for emergency use, developing a COVID-19 vaccine in collaboration with British pharmaceutical company AstroGeneca Oxford University. Serum, the world’s largest vaccine producer, is heavily leaning towards the AstraZeneca / Oxford vaccine because it can be stored at temperatures of 2 to 8 degrees Celsius, which is very cold for the Pfizer / Bioentech vaccine.
In the United States, health regulators met on Thursday to consider whether to approve the emergency use of the Pfizer / Bioentech vaccine and discuss another vaccine that US biotechnology company Moderna will develop after a second meeting a week later.
The world has reported more than 67.1 million cases of COVID-19, including more than 1.5 million deaths from the coronavirus. With 14.7 million cases and 282,312 deaths, the United States leads in both categories, according to Johns Hopkins University. [uh/ab]
–