Pfizer’s advanced countries have a large number of pre-purchases, so it is not easy to draw the time of introduction.
Janssen’s Phase 3 clinical trial completed in the first quarter of next year… Astrado has not yet been approved by developed countries.
Acceptance of’side-effect immunity’ requested by pharmaceutical companies
Chairman of the Medical Association “Difficulty in group immunity next year”
○ Pfizer vaccination,’first’ six months later than UK
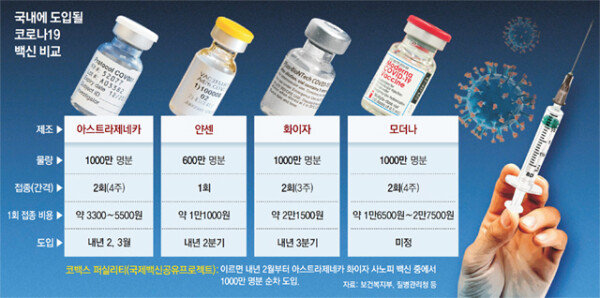
Prime Minister Jeong Sye-gyun emphasized on the 24th that “Jansen signed a total of 6 million people, which is 2 million more than the original planned volume.” It is positive that the company has secured additional vaccine volumes previously negotiated. However, the core of the recent issue surrounding vaccines is the timing of their introduction. ‘When will I get the vaccination?’ is also the biggest concern for the people. In terms of the timing of the introduction, some point out that they are disappointed with the contract of’Jansen in the second quarter and Pfizer in the third quarter.
The Pfizer vaccine has been evaluated as being the best match for the’safety’ emphasized by the government so far. Pfizer’s vaccine was the fastest in the world to start vaccination among several COVID-19 vaccines. Large-scale vaccinations are already in progress, led by the UK and the US. The most people are hitting in developed countries, so safety can be checked the fastest. However, it is first introduced in Korea in the third quarter. Given that the amount is supplied in stages, it may take a considerable period of time to complete the vaccination.
On the other hand, the Janssen vaccine, which the government has secured for 6 million people, has not yet completed a phase 3 clinical trial. I don’t know exactly how much prevention it has. The phase 3 clinical trial will be completed in the first quarter of next year. It is unknown whether it will end with a single dose. Janssen is currently conducting clinical trials up to two doses because there is a concern that the effectiveness of one dose will decrease. If the two doses are decided, the amount contracted by the government could be 3 million people. The AstraZeneca vaccine, which will be introduced from the first quarter, has not yet received emergency approval for use in developed countries. The Korean people first received a vaccine with relatively low safety. The government accepted the’side effect immunity’ in the same way as other countries that signed a vaccine contract. Lee Sang-won, director of epidemiological investigation and analysis at the Central Defense Countermeasure Headquarters, said, “It is a very special situation due to fierce competition to secure vaccines around the world,” he said. “There is a limit to holding manufacturers accountable as usual.” “Some of the vaccines contracted by the government will come in in the first half of next year, and some of the vaccines in the second half of next year are expected to be available in the second half of the year.” There is a high possibility that group immunity through vaccination will not be achieved.”
○ “It is difficult to advance the timing through additional negotiations”
It is said that the atmosphere of this negotiation process was generally unfavorable to the government. Among the government officials in the middle of the negotiations, it was even said that “the negotiations are becoming more disadvantageous if the high-spoken pharmaceutical companies have grasped the atmosphere of criticism in Korea.” It is said that the background that left room for further negotiations was due to cooperation between the government and domestic private pharmaceutical companies. In the KBS’Sunday Diagnosis’, Prime Minister Chung also appeared on the 20th, saying, “We are working diplomatically, but we expect that it will help to mobilize leading domestic bio companies that have a business relationship.
The government plans to accelerate the introduction of the vaccine as soon as possible through additional negotiations. However, the outlook is not so bright. The United States also mobilized the Defense Goods Production Act (DPA) to secure additional Pfizer vaccines produced in the country. As Pfizer said “the vaccine raw materials are insufficient,” the US government has signed an additional 100 million batches to provide raw materials. This could also affect the introduction of vaccines in other pre-purchase countries. Like domino, there is a possibility that there will be a disruption in domestic introduction. An infectious disease expert said, “The US will not try to give out vaccines made in their own country until the pandemic is over.” Even if it is a latecomer, the US NovaVax vaccine, we have to hurry up with a purchase contract.”
Joo-young Jeon [email protected], reporter Dong-woong Kang
Copyright by dongA.com All rights reserved.
—
–