Titular24 May. 2022
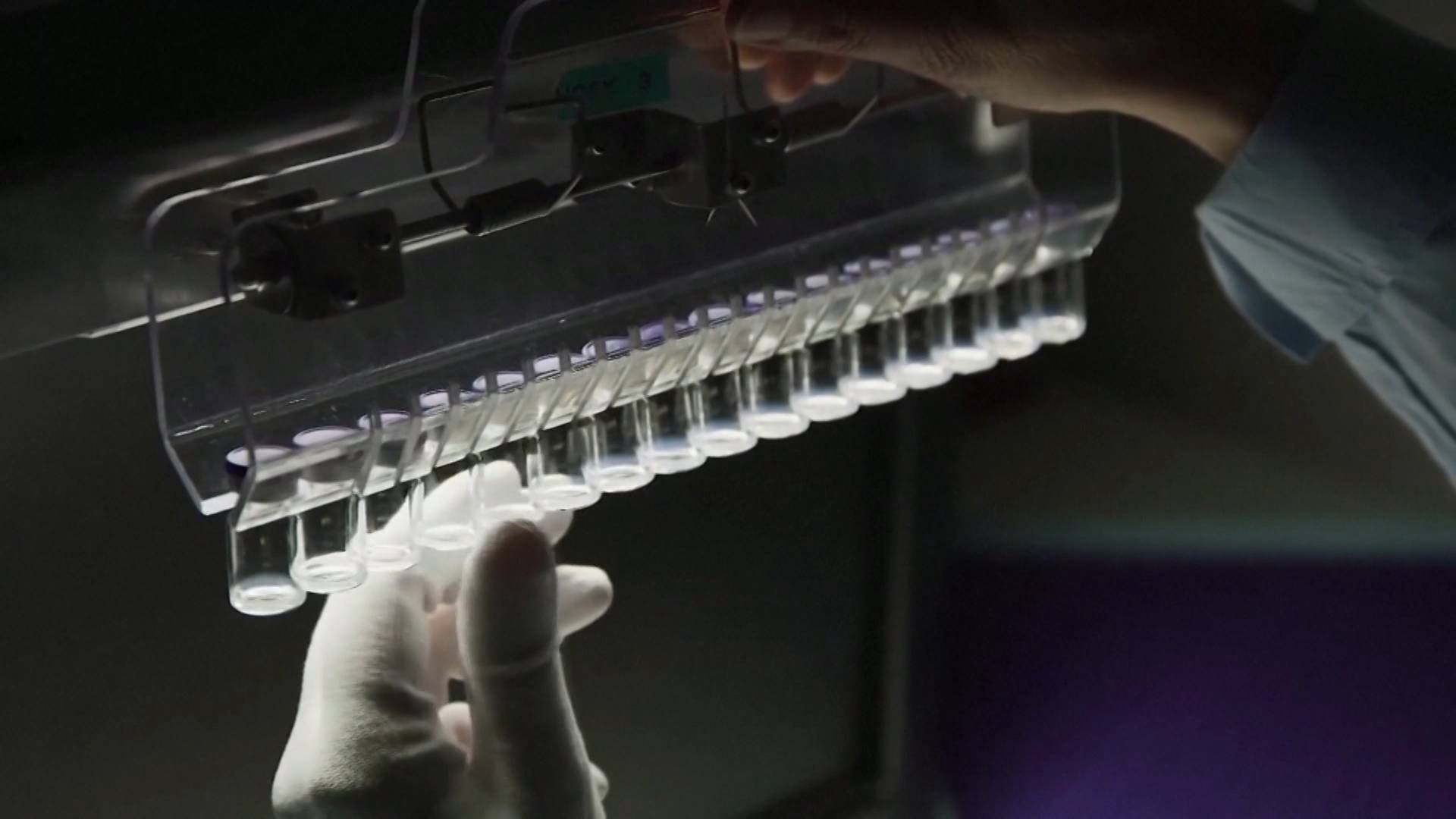
On Monday, Pfizer reported that three lower-content doses of its flu vaccine COVID-19 were safe and effective in preventing symptomatic diseases in children under 5 years of age. Pfizer’s announcement came as it prepared to file an emergency use application for its pediatric vaccine for children up to 6 months of age in the United States. The approval could come after the US Food and Drug Administration (FDA for its acronym in English) convene a panel of independent vaccine experts on June 15, the date on which said agency will also evaluate Moderna’s application for the emergency use of its vaccine in minors.
—
/data/photo/2022/05/23/628aa06349506.jpeg)
