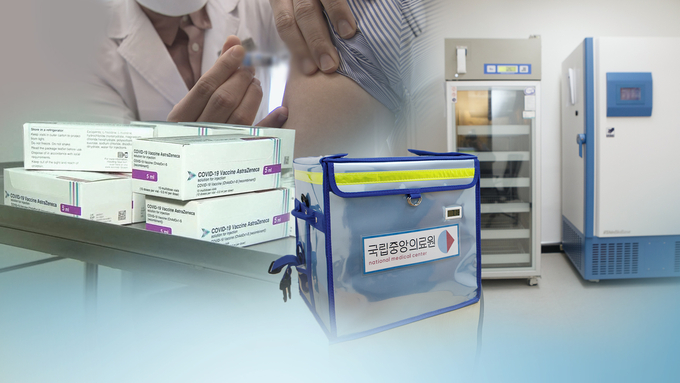
As the government decided to vaccinate the novel coronavirus infection (Corona 19) sequentially starting this month, the issue of whether to allow the AstraZeneca (AZ) vaccine to the elderly aged 65 or older has emerged as a key issue.
If a decision is made to allow AZ vaccination for the elderly, a large-scale vaccination plan can be promoted without major disruption, but if a decision to disapprove is made, a major revision of the plan itself is inevitable.
◇ Controversy over the effect of vaccination of the elderly with AZ vaccine in Europe… Influence in Korea
The AstraZeneca vaccine is at the center of controversy because the controversy over the effectiveness of vaccination for the elderly in Europe is increasing.
According to an announcement from the quarantine authorities and each country on the 6th, the European Union (EU) officially approved the conditional sale of the AstraZeneca vaccine on the 29th of last month, but Germany and France were under the age of 65 because there were insufficient data to prove the efficacy of the vaccine to the elderly. Recommended for vaccination only.
In addition, Belgium has lowered the age of those who are vaccinated to under 55.
Italy initially recommended preferential use for people under the age of 55, but recently revised its opinion that even over the age of 55 can get the vaccine if they are healthy.
Although not a member of the EU, the Swiss government has withheld approval for the AstraZeneca vaccine and demanded that additional data be submitted, and Norway has officially announced that it will not vaccinate people over 65.
◇ Among the three steps of the Ministry of Food and Drug Administration’s consultation process, the first step is’Vaccination is possible’, and the second step is’Judgement reservation… Final decision by the Agency for Disease Control and Prevention
This controversy is having a direct impact on Korea as well.
The day before the Ministry of Food and Drug Safety, as a result of the Central Pharmacist Review Committee (Central Pharmacy) meeting, the second expert advisory stage for AstraZeneca vaccine, AstraZeneca vaccine vaccination is permitted to those aged 18 and over, but for those aged 65 or older, the KCDC prevents later. The vaccination expert committee (Vaccination and Vaccination Committee) recommended discussion.
“There is not enough data on the effectiveness of AstraZeneca vaccination over the age of 65, so it must be decided carefully,” the Central Pharmacist concluded.
It did not make a judgment on whether or not to be vaccinated, and turned over the responsibility for the final judgment to the KMA.
The Ministry of Food and Drug Safety is undergoing a’triple’ expert advisory procedure leading to the COVID-19 vaccine safety and effectiveness verification advisory group (verification advisory group), the Central Pharmacopoeia, and the final inspection committee.At the first stage verification advisory group meeting held on the 1st,’Including the elderly ‘Vaccination for all age groups’ was the majority opinion, but the second stage Central Pharmacopoeia concluded that it was’deferred judgment’.
◇ If vaccination for the elderly with AZ vaccine fails, the entire vaccination plan is disrupted
The KFDA issued a statement immediately after the decision of the Ministry of Food and Drug Safety and the Central Pharmaceutical Affairs Deliberation the day before, “reflecting the results after the final approval and examination by the Ministry of Food and Drug Safety, and reviewing the’Corona 19 Vaccine Expert Advisory Group’ and reviewing the’Vaccination Specialized Committee’. After that, we will confirm the AstraZeneca vaccination plan for the elderly over the age of 65.”
However, amid controversy in Europe, even the Chinese medicine center raises the need for a careful decision, so it is expected that the Agency for Disease Control and Prevention will not be able to draw conclusions easily.
Moreover, if the schedule of the final inspection committee, which is the final stage of deliberation by the Ministry of Food and Drug Safety, has not been specified, and the decision on the vaccination committee by the Korean Disease Service is required, the time remaining until the start of vaccination at the end of this month is very short.
Vaccination can be disrupted from the start.
From the end of this month, the government has set up a plan to vaccination against medical staff such as doctors, nurses, hospital workers, etc. who care for Corona 19 patients, and residents of nursing hospitals and facilities with a large number of elderly people over 65 years old.
Among them, it is known that the corona 19 medical staff are almost caught receiving the Pfizer vaccine secured through the’COVAX facility’, an international project for joint purchase and distribution of vaccines, and the elderly receive the AstraZeneca vaccine.
If it is decided that AstraZeneca vaccine cannot be vaccinated for the elderly, the initial vaccination plan will have to be wrong. In this case, only the medical staff will receive the vaccination first, and for the elderly, the vaccination may have to be delayed until another vaccination such as Modena comes in.
Currently, the timing of introducing vaccines directly supplied by each pharmaceutical company in Korea is from Q1 for AstraZeneca (for 10 million people), Janssen (for 6 million people) and Modena (for 20 million people) from Q2, and Pfizer (for 10 million people). Are held from 3Q.
Even if the AstraZeneca vaccine is decided to inoculate the elderly, controversy over its effectiveness is likely to continue, and disruption may occur in the vaccination process as well.
In the end, the first vaccination was completed for all citizens by September in the order of seniors at medical staff and nursing hospitals in the first quarter, seniors aged 65 years or older in the second quarter, and adults aged 18 to 64 in the third quarter. It is also impossible to rule out the possibility that the entire vaccination plan to form’will not be able to cruise.
◇ “If vaccination for the elderly, the vaccination plan will be messed up”, “There is no adverse reaction in the UK, so there will be no disruption in domestic schedule”
One expert who asked for anonymity said, “The original AstraZeneca vaccine was to be given priority to those in the elderly, nursing hospitals and nursing facilities before other vaccines. If the vaccine cannot be given to people over 65, the vaccination plan is messed up. “The Vaccination Committee is not an organization that decides whether or not to approve drugs, and it is a matter to be decided by the Ministry of Food and Drug Safety,” he said.
According to data from the UK, where vaccination has already started for the elderly, many opinions have suggested that there will be no major disruption to the domestic vaccination schedule.
Jeong Jae-hoon, a professor of preventive medicine at Gachon University Medical School, said, “It seems that the Ministry of Food and Drug Safety is waiting for evidence (data) from a very cautious standpoint. In the UK, vaccinations are already performed for the elderly, and in about a month (at the time the domestic vaccination begins) “I think the data will accumulate.”
Professor Jeong said, “It is important that the UK and European authorities have approved the AstraZeneca vaccine,” he said. “There is an alternative to Pfizer vaccine in countries that are cautious about vaccination with AstraZeneca, but since Korea does not have an alternative, we calculate the risks and benefits. When you do, you have to start the vaccination somehow.”
Professor Kimoran of the National Cancer Center also said, “We have already begun vaccinating AstraZeneca on a large scale in the UK, and there are many people who have actually been hit,” he said. “If there was an adverse reaction, it would have been reported, but there was no such report.
Professor Ki explained, “The safety issue should be considered as an adverse reaction. In the clinical trial of (AstraZeneca Vaccine), there was a concern that there were few elderly subjects, but the safety was confirmed as there are currently no reports of adverse reactions.” did.
– .