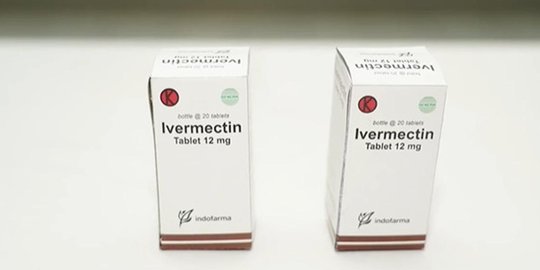
Merdeka.com – The University of Oxford has launched a clinical trial to test whether Ivermectin is effective in treating the disease caused by the corona virus infection or Covid-19. This drug is usually used to treat diseases caused by parasitic infections, including onchocerciasis or ‘river blindness’ due to the nematode worm Onchocerca volvulus.
But the use of Ivermectin to treat Covid is controversial, and the European Drug Administration (EMA) advises against using it outside of randomized clinical trials.
Despite being promoted as a cure for Covid in some countries, such as Brazil, most scientific studies of its effectiveness involve poorly controlled experiments or small numbers of participants. There is little evidence of the drug’s efficacy.
Research shows Ivermectin can kill the SARS-CoV-2 coronavirus in laboratory-grown cells and in live mice (but only at high doses that can be toxic to humans), while observational studies of people already taking the drug suggest it may be effective for Covid. — but the study did not control for confounding factors such as when people deliberately choose drugs, leading to problems such as the placebo effect.
Quoted from the Forbes page, Thursday (24/6), the Oxford large-scale trial will involve giving Ivermectin to parents and adults who represent a history of the disease. By comparing these participants with patients receiving standard care from the UK’s National Health Service (NHS), the study aimed to reveal whether the drug helped people get out of hospital.
Like other well-known drugs, including hydroxychloroquine and Remdesivir, people are currently using Ivermectin for purposes not specified on the drug label. Such off-label uses include a waste of money if the drug is ineffective, and potentially dangerous if someone uses the drug instead of a proven drug.
The Oxford study is part of a broader project called PRINCIPLES (Platform for randomized community trials of treatments for epidemic and pandemic diseases), which is trying to find treatments for Covid-19 from home by recruiting participants via its website and patients from practicing physicians. . [pan]
– .
/i/1291905847.png?f=fpa)
