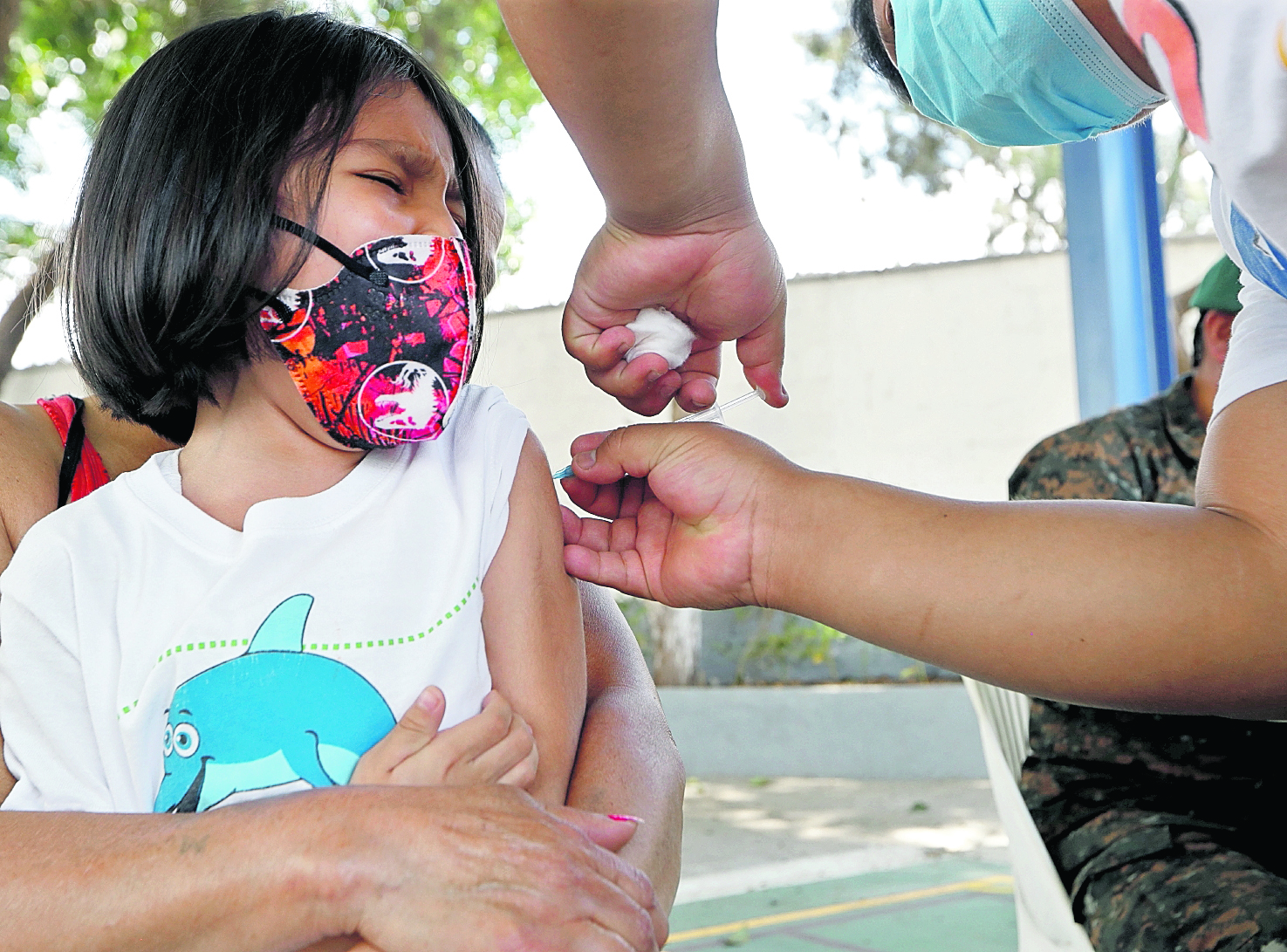
Twenty-eight days is the deadline that Moderna established for the second dose of the covid-19 vaccine for children between 6 and 11 years old, according to Asofarma, which became the link between the pharmaceutical company and the Central American countries to distribute SpikeVax, trade name of the biological.
“Moderna and Asofarma recommend that these intervals be respected since they are the ones tested in controlled clinical studies of efficacy and safety”, they responded to the query made by Free Press about the time that can elapse to complete the vaccination schedule in children.
–
However, in Guatemala, the period for that second dose was extended to 56 days, a time that is fulfilled on May 5 for the first children who received the biological in Huehuetenango on March 11. The dilemma is that the authorities maintain the discourse that the biological will be available to complete the regimen for these children, but to date there is nothing concrete about the purchase of doses.
According to Dr. Alicia Chang, president of the Guatemalan Association of Infectious Diseases (AGEI), there are studies that support the placement of the second dose of Moderna to reduce the incidence of adverse events such as pericarditis, but in children between 6 and 11 years there are no scientific documents to support it.
This is information that was extrapolated from a study that was carried out in adolescents -between 12 and 17 years old-, who did benefit from receiving the dose at two months, but extending more than this period is not recommended “because what one wants is to have a complete immunization as soon as possible”, says Chang.
Outside that time it is not convenient because the vaccination of a population group that is still exposed to covid-19 is unnecessarily delayed, especially when a new variant circulates in the country, since the vaccines that are currently administered are not as effective against omicron as for the other variations, such as delta, says the expert.
“The World Health Organization through its group of immunization experts recommend that, if for any reason the second dose is delayed beyond the 28-day interval, it should be applied as soon as possible to complete the immunization schedule. ”, says Asorfarma.
No certainty of second doses
As for whether Guatemala has already made any negotiations with Asofarma for the acquisition of vaccines from Moderna, Bernardo Girala, General Manager of the company for Central America and the Caribbean, indicates that “approaches have already been made and we are waiting for a response for a appointment with the authorities”, but there is still no talk of buying the biological.
The only way out that the country has at the moment is that there is a donation of vaccines from Moderna to continue with the inoculation of children, but it is something that has not been confirmed.
The National Council for Immunization Practices (Conapi) recommended starting to vaccinate children until they are guaranteed a complete scheme, however, the suggestion was not binding, and President Alejandro Giammattei announced on March 11 the process of inoculating children between 6 and 11 years without having that guarantee, without taking into account the opinion of the experts.
Chang says that not having the two doses for the children was a weak point that the AGEI saw from the beginning of the process. “We knew that this second dose was not going to be available in time,” she says, mainly because the acquisition of the biological has been a complication for the country since the first vaccines against covid-19 emerged.
Waiting for another donation from the US government of Moderna vaccines for Guatemala and for them to be applied to children between 6 and 11 years of age is a distant possibility for Chang. “The United States has been very clear that they cannot donate a vaccine for use in children if they have not approved it,” because so far the FDA has not given the green light for the emergency use of said biological in this population segment. , only Pfizer approved.
“If that approval does not exist, and if the expectation is that it came from the United States, this could not happen,” warns the infectologist.
Is there a possibility to combine them?
Moderna through Asofarma indicates that they have not conducted clinical studies with the use of other available vaccines against covid-19, “therefore it does not recommend heterologous immunization schemes.”
Mixed vaccination schemes -combination of brands- are not approved for children under 12 years of age, have not been studied and, according to the president of the AGEI, will not happen in the short term, since the manufacturers are focused on analyzing the effectiveness of two doses of its own brands.
The experts warned when the vaccination campaign began in children, that if they received a dose of Moderna and the country managed to acquire doses of Pfizer, the minors should receive the second injection of Moderna, since there is no scientific evidence to support otherwise. .
Eliú Mazariegos, director of the Comprehensive Health Care System -Sias-, indicates that the office of the minister, Francisco Coma, is taking steps to guarantee the second dose. “From the normative technical point of view, there are 56 days to administer the second dose. Ideally, it should be administered within that period, ”he mentions.
While the deputy Evelyn Moraratya refers that it is unacceptable that the vaccination process has begun in children without having ensured that they complete the scheme against the coronavirus. The situation is not new, as it also occurred at the beginning of the adult immunization process, when the second component of Sputnik V was slow to arrive. The time to apply the second dose was extended as the delay became more evident, from a period of 21 days, it was changed to 61 and later to 90 days. It seems that the same thing happens with the inoculation of minors, at the discretion of the legislator.
–