–
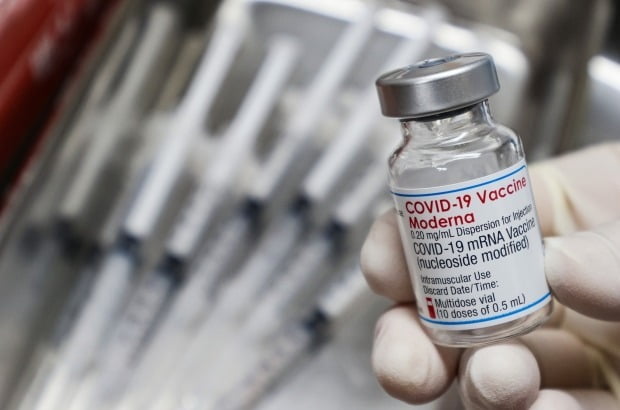
The Ministry of Foodstuff Security and Medicines (MFDS) has approved Moderna’s new bivalent vaccine for coronavirus (COVID-19) an infection “Moderna Spikebox 2 Months”, developed to also respond to Omicron mutations.
The Ministry of Foods Safety and Medicine declared on the 8th that it experienced determined to approve the product or service after carefully examining the results of the safety, efficacy and good quality critique and expert information for this vaccine. Even so, soon after acceptance a ailment was hooked up that lengthy-expression retention take a look at facts to fix the expiration date should really be submitted.
According to the Ministry of Food stuff Protection and Medications, Moderna Spikebox 2 is a bivalent vaccine formulated to concurrently react to the preliminary Corona 19 virus and the Omicron “BA.1” mutant virus.
Moderna Korea experienced earlier applied to the Ministry of Foods Protection and Medication for a preliminary evaluation and posting acceptance for this vaccine in July.
The efficacy and influence are the avoidance of COVID-19 in subjects around 18 years of age and the dosage and administration is .5 ml (50㎍) of booster injection at least 3 months after receiving the most important or booster inoculation.
The govt plans to initiate even more vaccinations for the elderly and immunocompromised with this vaccine starting off Oct as early as Oct.
As a consequence of evaluating the immune response of this vaccine with that of the preceding vaccine, the volume of neutralizing antibodies was 1.22 times higher for the original Corona 19 virus and 1.75 situations larger for the Omicron mutation, the Ministry of Stability claimed. food and drugs.
This vaccine has already obtained conditional acceptance in the European Union (EU), United kingdom, Switzerland and Australia and is utilised as a booster injection.
The Ministry of Food Security and Medications stated: “We will do our ideal to improve the monitoring method for irregular situations right after vaccination in cooperation with the appropriate ministries and will do our most effective to make certain that the public can be vaccinated with self-confidence through complete checking and well timed reaction. “
He added: “Upon acceptance of the domestic shipment, we will evaluate the manufacturer’s creation and examination final results and conduct direct checks to verify the quality of the solution.”
Meanwhile, the Ministry of Food Security and Medicines is also searching into merchandise acceptance for Pfizer’s BA.1-dependent bivalent vaccine.
Reporter Lee Bo-bae, Hankyung.com [email protected]
–

![[국제]”Declaration of war on Japan” … “Emergency” of Japan owing to a professional-Russian hacker attack [국제]”Declaration of war on Japan” … “Emergency” of Japan owing to a professional-Russian hacker attack](https://image.ytn.co.kr/general/jpg/2022/0908/202209082319510500_t.jpg)