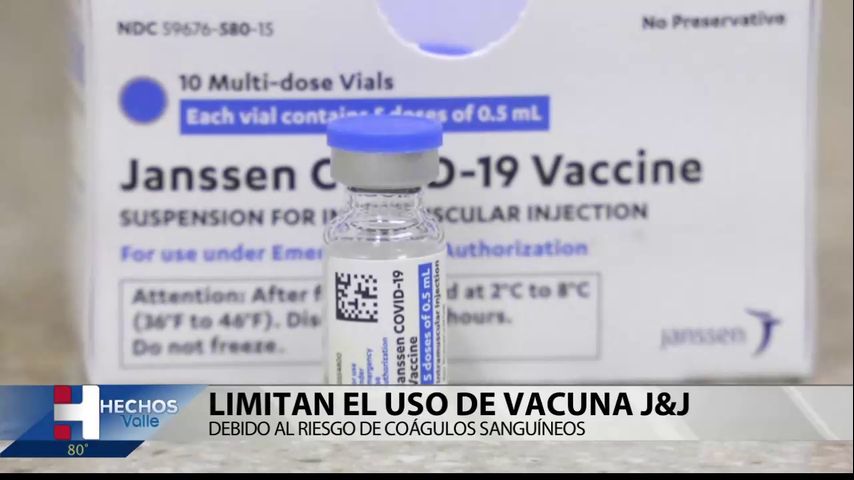
The FDA announced that it will limit the emergency use authorization of Johnson & Johnson’s COVID-19 vaccine to people 18 years of age and older.
In an FDA statement, it was reported that the change is motivated by the risk of a rare and dangerous clotting condition called thrombosis with thrombocytopenia syndrome, which can occur after receiving the vaccine.
The agency confirmed that the updated authorization also applies to booster doses.
–
–
Related posts:
Preventing Childhood Pneumonia: Importance of Vaccination and Steps to Take
RAISA announces the achievement of 2020, "Thai Can Do", the outstanding innovation of COVID vaccine ...
'Forestall mastitis in dairy cows' Kim Jae-myeong, head of the bacterial illnesses division on the H...
This is what the new corona rules in RLP bring with them - SWR Aktuell
/data/photo/2022/05/07/62766f677e3cf.jpg)