The International Association of Innovative Pharmaceutical Companies has compiled data for the three Baltic States, and its director, Valters Bolēvics, points out that although there has been moderate growth in the state-funded cancer drug system over the past year, the availability of medicines for cancer is changing slowly.
In addition, many patients in Latvia need special treatment, even medicines that are not registered in Latvia, not to mention the list of reimbursable medicines. Doctors’ councils determine the necessary treatment by assessing the individual situation of each patient and the cancer research performed at the patient’s own expense, but the National Health Service is forced to refuse support by refusing to pay for drugs because the state already lists some cancer drugs. Patients receive such a refusal even if doctors indicate that the listed, state-funded therapy does not help. The charity portal ziedot.lv has several requests from patients to donate to cancer drugs.
Neighbors are moving forward
In Lithuania, the number of innovative medicines is 32 percent higher – 99 younger generation medicines are paid for by state patients, while in Estonia the number of state reimbursable medicines is 16 percent higher than in Latvia, and 80 innovative medicines are available for Estonian patients, according to the International Association of Innovative Pharmaceutical Companies. the number of state-paid innovative medicines in the Baltic States in September 2020. Comparing the indicators with the neighboring countries, Latvia has the lowest number of state – paid innovative medicines for the treatment of patients – 67.
–
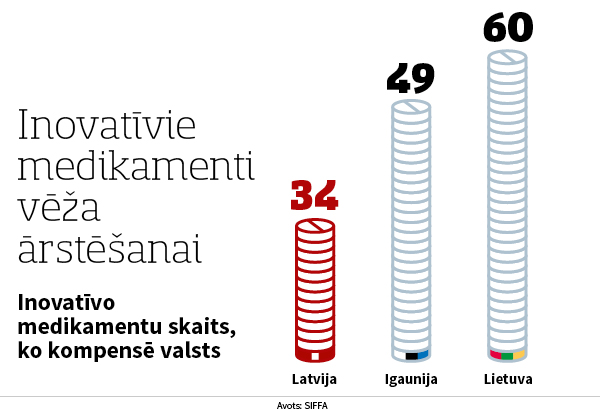
The situation is similar with innovative medicines directly for the treatment of cancer. During the six months, the list of reimbursable medicines in Latvia has been supplemented by three new innovative medicines for the treatment of cancer, and thus the list includes 34 medicines. However, there are 60 medicines for oncology in Lithuania and 49 in Estonia. These data confirm the dire situation that oncology patients in Latvia receive significantly less funding for new and effective medicines than in other Baltic countries, says the director of the association Valters Bolēvics.
Patients survive with medication
Cancer is one of the leading causes of premature death in the European Union, but 40% of cancers can be prevented by changing approaches to treating the disease. The European Commission has defined the fight against cancer as one of the main priorities, being clearly aware of the benefits it can bring to patients and the country as a whole, but the situation in Latvia is different.
This is also indicated by the waiting time of Latvian patients for innovative medicines for the treatment of this disease –
981 days, or almost three years, have elapsed since the registration of a new medicine on the market (at the European Medicines Agency),
data from the European Federation of Pharmaceutical Manufacturers and Associations for 2015-2018 show period of one year.
 –
–
–
“Innovation in any field ensures development, it is a rather basic and logically understandable basic principle,” V. Bolevics believes. “A significant example is Covid-19 and the role of pharmacy in combating it, where scientists have been able to develop antiviral vaccines and drugs for less than a year.” The association believes that in order to improve the availability of medicines for Latvian patients, it is necessary to purposefully move towards an annual increase in funding for reimbursable medicines, especially in the field of oncology. “Planned and annual increases for reimbursable medicines are a combination of two key words that should be followed so that the state can plan how many new medicines and what diagnoses to list each year, and we cannot ignore developments in the pharmaceutical sector that are moving in a completely different direction, that is, the treatment of people at the gene and cellular level, ”emphasizes the head of the association.
Read the story of Elena Rituma, a breast cancer patient here.
OPINION
 – –
– –
Valters Bolēvics, Director of the International Association of Innovative Pharmaceutical Companies:
– Despite moderate growth, we are evaluating the addition of at least three innovative medicines to the list of reimbursable medicines. Following the innovations in the industry, scientific progress and patient needs, the growth rate is still too slow, but at the same time it should be noted that significant progress has been made over the last three years, with more than doubling the number of innovative medicines. Latvia does not have to make financially intensive investments in the invention and production of medicines – the only homework that the state has to do is introduce a more accessible and sustainable health care system so that the country can afford its newest and most effective medicines that undoubtedly provide scientifically proven results.
–


