An example of an electrolytic cell is one application of an electrochemical cell. Electrolytic cells can convert electrical energy spontaneously into chemical reactions.
Metal is a type of material that rusts easily. To be able to remove the rust, then metal plating can be one solution.
Metal plating occurs by coating a metal surface with another metal through an electrolysis process. For example, a knife coated with silver will resist rust.
Also Read: Difference Between Voltaic Cell and Electrolytic Cell and Their Applications
Definition and Examples of Electrolytic Cells
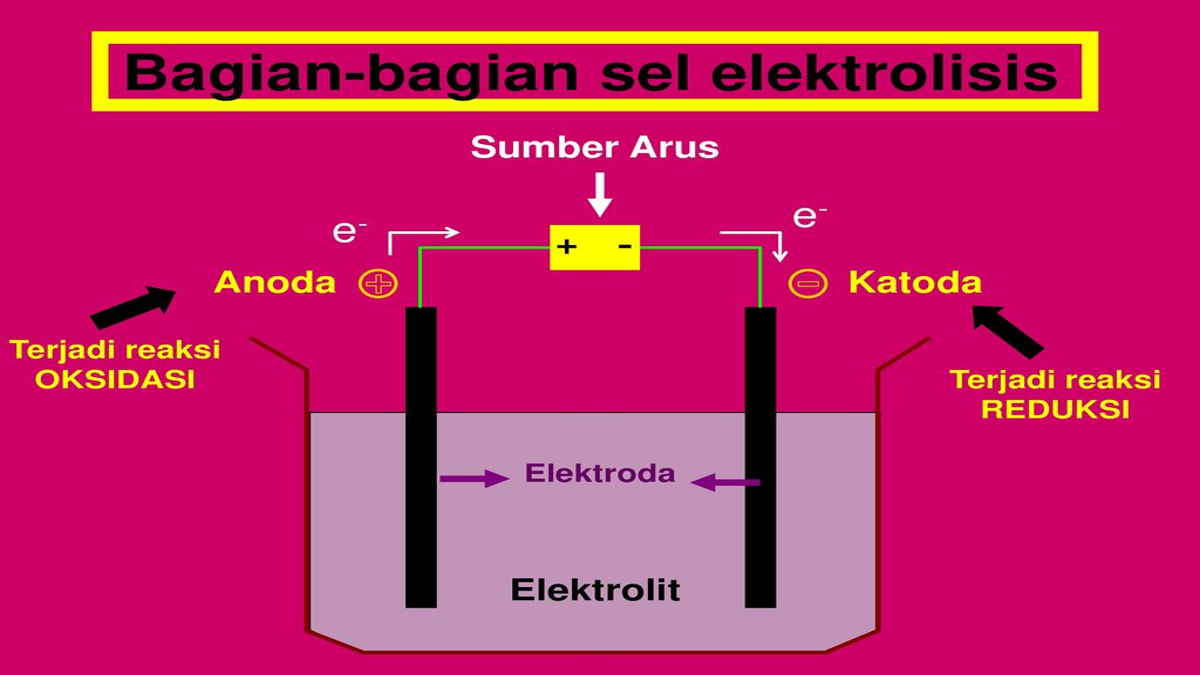
The electrolytic cell is one of the electrochemical cells. In this cell, electrical energy will be useful for carrying out redox reactions that are not spontaneous.
Then the electrolysis reaction has a definition as a reaction from the decomposition of substances using an electric current. In general, an electrolytic cell is composed of:
- The power source in charge of supplying direct current or Direct Current (DC), for example a battery.
- The cathode is where the reduction reaction takes place (negative pole).
- Anode which is the electrode where the oxidation reaction occurs (positive pole).
- Electrolytes are substances that conduct electrical energy.
Electrons will flow from the cathode to the anode. Then, the positive ions will tend to be attracted towards the cathode and be reduced, while the negative ions will tend to be attracted towards the anode and oxidized.
Here are two examples of cells that you can understand.
Also Read: Faraday’s Law of Electrolytic Chemistry: Definition, Types and Formulas
Electroplating or Plating
The first example of the use of an electrolytic cell was as the plating of metallic silver from a solution of silver nitrate.
This method aims to coat certain metal surfaces with silver. This will make them have certain properties so they are not easy to rust and are stronger.
In this application, the metal that is going through the plating process will be placed as the cathode and must have a negative charge from the electric current.
Because it is coated with silver, the cathode will be silver and the electrolyte, which is silver nitrate solution.
As already mentioned above, an example of a plating electrolysis cell is coating a knife with silver. That way, the knife will last longer.
Also Read: Electrolyte and Non-Electrolyte Solutions, Characteristics, Examples, and Differences
Aluminum Electrolysis
The next example is the process of electrolysis of aluminum which is in the process of making aluminum and also its ore.
This process takes place with carbon electrodes that have inert properties or do not react easily which will act as anode and cathode.
When the electric current begins to flow, then in the Al2O3 electrolyte there will be a reaction of reduction and oxidation.
Then, at the anode in the form of carbon, an oxidation reaction will occur and at the cathode in the form of carbon, there will also be a reduction.
The aluminum resulting from this process will be in solid form and will settle and stick to the carbon cathode.
Well, that’s the understanding as well as an example of an electrolytic cell in life. Without us realizing it, the existence of electrolysis cells as a chemical process is very useful in this life. (R10/HR-Online)
–