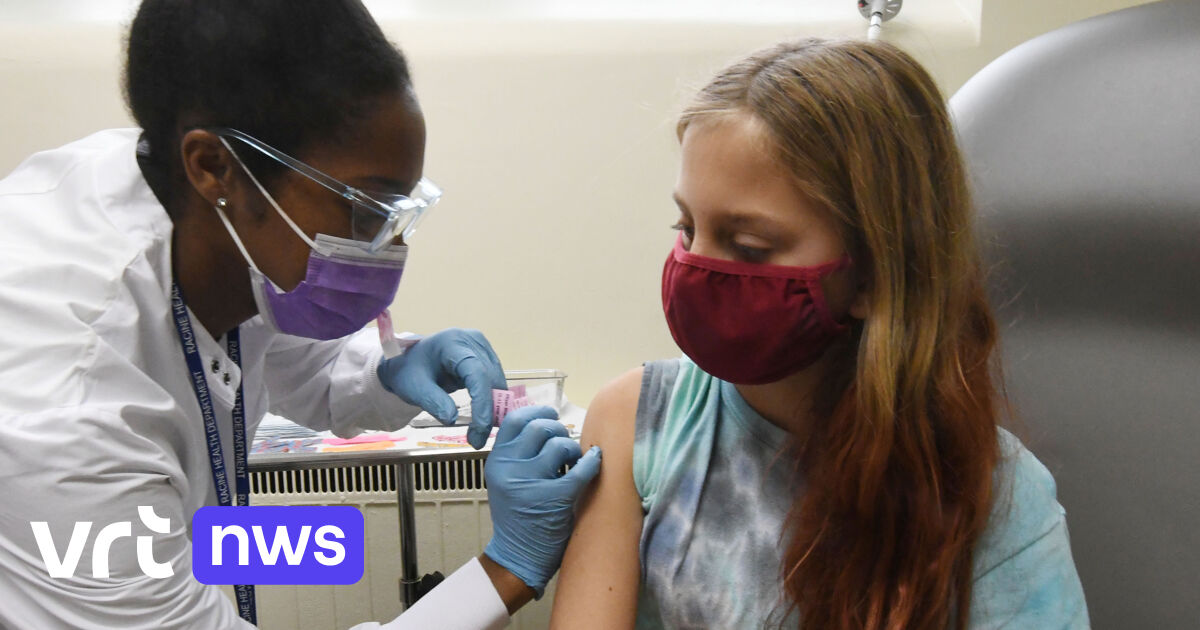
Children are treated with a reduced dose of 10 microgram, compared to 30 micrograms for adults. As with adults, they receive two injections in the upper arm, three weeks apart.
Pfizer conducted a study with some 2,000 children, and concluded that the vaccine has a 90.7 percent effectiveness against symptoms from day 7 after the second dose.
The clinical tests showed that children had fewer side effects after the injection. Serious side effects have not been identified. The test group is too small to notice rare side effects, says Pfizer. The most common side effects from the study were the same as those seen in adults, including some pain at the injection site, fatigue, and possible feverish symptoms.
–