EU Commission, final approval immediately after recommendation for approval by the European Drug Administration
On the 6th (local time), EU Commissioner General Ursula Ponder Lyen tweeted, “We have approved Modena vaccine. It is the second vaccine approved by the EU.”
“We are providing a safe and effective COVID-19 vaccine to Europeans,” he said. “Europe has potentially secured 2 billion doses of vaccine. It is enough to protect all of us.”
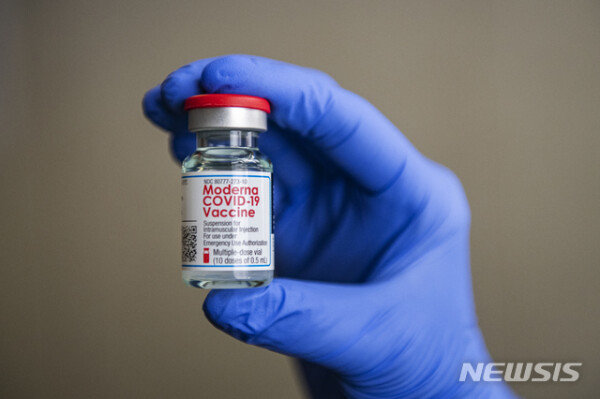
The EU Commission issued a permit a few hours after the European Medicines Agency (EMA), which is responsible for evaluating and approving vaccines, recommended conditional approval for modders or vaccines. In a trial, it said it was effective in preventing COVID-19, and said it recommends approval for conditional use for people over the age of 18. This is the second time the EU has approved a COVID-19 vaccine.
The EU approved the COVID-19 vaccine jointly developed by US pharmaceutical company Pfizer and German Bioentech on the 21st of last month, and a week later began vaccination in 27 member countries.
[런던=뉴시스]
Copyright by dongA.com All rights reserved.
—
–

:quality(80)/cdn-kiosk-api.telegraaf.nl/8fffe1ba-5014-11eb-821c-02d2fb1aa1d7.jpg)