theme image — © REUTERS
–
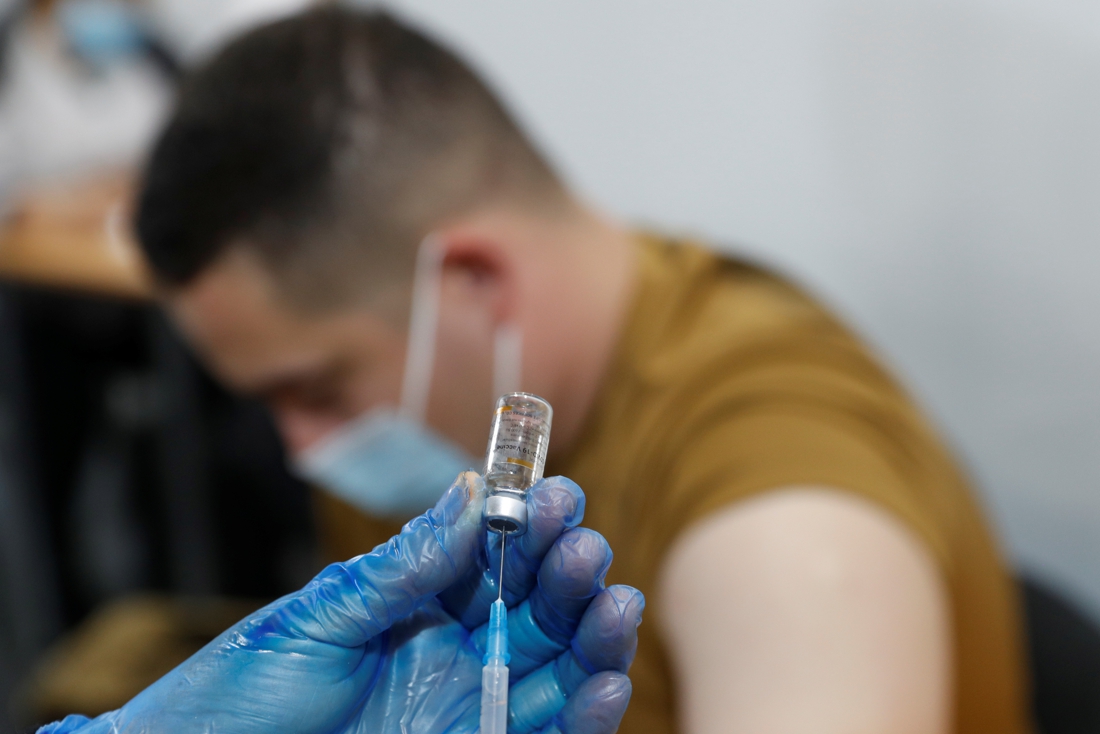
For a third shot against the coronavirus, the so-called ‘booster shot’, better use is made of the vaccine that was used for the first vaccination. This is reported by the European Medicines Agency EMA. That way, a stronger immune response would be elicited than a third shot of the same vaccine.
–
jvhSource: Belgian
—
The US drug agency FDA recently approved this method, and the EMA says it is currently studying the necessary data to decide whether it agrees with that advice. In the European Union, only the vaccines from Pfizer/BioNTech, Moderna, Johnson & Johnson, and AstraZeneca are currently approved for a first vaccination, for a third shot, only the Pfizer vaccine has been approved for the time being.
“We see some promising research results confirming that this approach elicits a stronger immune response with certain combinations of vaccines than when the same vaccine is used for an additional injection,” said Marco Cavaleri, EMA’s vaccine strategy director at a news conference on Thursday.
Johnson & Johnson
A study published last week in the United States found that people who received the Johnson & Johnson vaccine — which uses classic viral vector technology — seemed to be better protected if they received a booster dose of an mRNA vaccine such as Pfizer or got modern. The latter “seem to work much better” at boosting the immune system, and “are able to elicit a really strong immune response,” Cavaleri said.
The EMA will decide on 25 October whether booster shots with the Moderna vaccine will also be approved.
— .