Input 2021.01.02 11:16
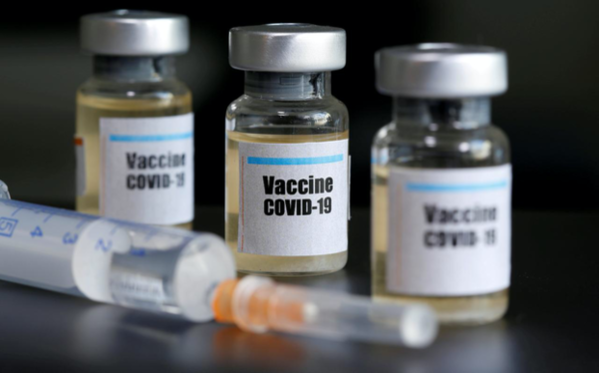
In particular, test methods necessary for testing and testing of mRNA vaccines using new technology will be established in advance before application for approval. To this end, it is preparing to proceed with national shipment approval, such as urgently purchasing 9 types of analysis equipment and securing an analysis room dedicated to RNA.
The Ministry of Food and Drug Safety aims to shorten the approval period for existing products that took more than 180 days to less than 40 days through preliminary review and approval review by item for corona vaccines and treatments.
–
–
The Ministry of Food and Drug Safety has been operating a’high-intensity rapid commercialization promotion program’ (GO-rapid program) that includes this content since April last year. To support the development of vaccines and treatments.
The Ministry of Food and Drug Safety has also established a’team review operating system’ to thoroughly review applications for approval and clinical trials of corona vaccines and treatments. By type, there are’Virus Vector Vaccine Team’,’Nucleic Acid Vaccine Team’, and’Antibody Treatment Team’.
From 90 days before the application for product permission is expected, the’Permission Deliberation Team’ composed of expert reviewers in each field is formed to conduct preliminary consultation and review before applying for permission.
The Ministry of Food and Drug Safety has newly established the’Expedited Review Division’, an organization dedicated to the rapid review of corona drugs. In addition, the’Expert Council’ has been formed and operated to consult external experts for the expertise and transparency of the examination.
–
– .

:strip_icc():format(jpeg)/kly-media-production/medias/3327813/original/031204100_1608289370-Mi_10T_Pro.jpg)