Outer space is rich in carbon dioxide (CO2). During this time on the Space Station (ISS), the compound was captured so as not to be inhaled by the astronauts. The resulting carbon dioxide is captured and converted to methane (CH4) as a booster rocket fuel to keep the station in high orbit.
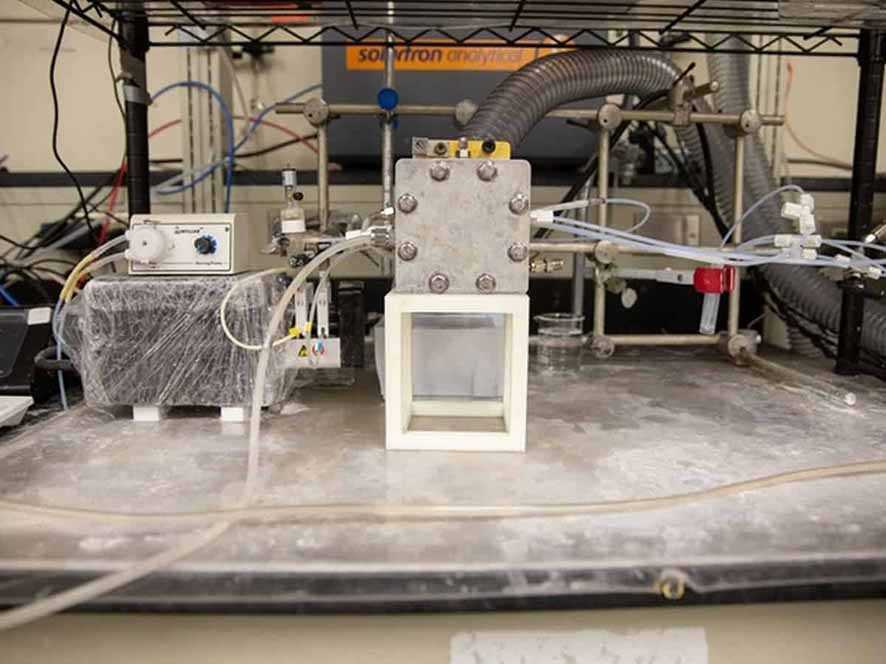
Recent research conducted by engineers at the University of Cincinnati, Ohio, United States (US), succeeded in developing carbon dioxide into methane using only the reaction method of the French chemist, Paul Sabatier, with a carbon catalyst.
Jingjie Wu, assistant professor at the University of Cincinnati’s UC College of Engineering and Applied Science, and students say carbon catalysts can convert carbon dioxide into methane. By reacting in a Martian atmosphere composed almost entirely of carbon dioxide, astronauts could save half the fuel needed for a return trip to Earth.
“It’s like a gas station on Mars. You can easily pump carbon dioxide through this reactor and produce methane for the rocket,” Wu told the University of Cincinnati website.
The study, published in the journal Nature Communications in the Thursday issue of last week with collaborators from Rice University, Shanghai University and East China University of Science and Technology, was originally conducted to study fuel cells for electric vehicles. But then Wu instead switched to converting carbon dioxide into methane.
“I realized that greenhouse gases would be a big problem in society,” Wu said. “Many countries realize that carbon dioxide is a big problem for the sustainable development of our society. That’s why I think we need to achieve carbon neutrality,” he said.
Wu explained that the administration of US President Joe Biden has set a target of reducing greenhouse gas pollutants by 50 percent by 2030. In addition, by 2050 all economic activities will use fully renewable energy. “That means we have to recycle carbon dioxide,” Wu said.
Develop Catalyst
Currently Wu and students such as doctoral candidate at the University of Cincinnati, Tianyu Zhang, are experimenting with various catalysts such as graphene quantum dots. The catalyst is in the form of a nanometer-sized carbon layer that can increase the amount of methane produced.
The process of converting carbon to methane on Earth could help mitigate climate change. But it also has a great commercial advantage in producing fuel as a by-product.
“The process is 100 times more productive than 10 years ago. So you can imagine the progress will be faster,” said Wu. “In the next 10 years, we will have many start-ups to commercialize this technique,” he said.
Zhang used a different catalyst to produce not only methane but also ethylene. The latter product is commonly used in the manufacture of plastics, rubber, synthetic clothing, and the like. “Green energy will be very important. In the future, it will represent a very large market. So I want to work on it,” Zhang said.
In synthesizing fuels from carbon dioxide to become more commercially viable, it needs to be combined with renewable energies such as solar or wind power. According to Wu, the world currently has a surplus of environmentally friendly energy that has unfortunately been thrown away. Though this renewable energy can be stored in the form of chemicals.
Carbon conversion can be done in diesel or coal power plants. In a day in that place produces tons of carbon dioxide. According to Wu, the conversion process where carbon dioxide is produced is more efficient.
While the application of this technology on Mars makes astronomers sure they can set foot on Mars and can return to Earth. “Right now if you want to come back from Mars you need to carry twice as much fuel and that’s very heavy.” hi/I-1
Also Read:
Twitter Fleet Feature Has Been Removed, Lasts Only 8 Months
–
–
Editor : Ilham Sudrajat
Writer : Haryo Brono
– .