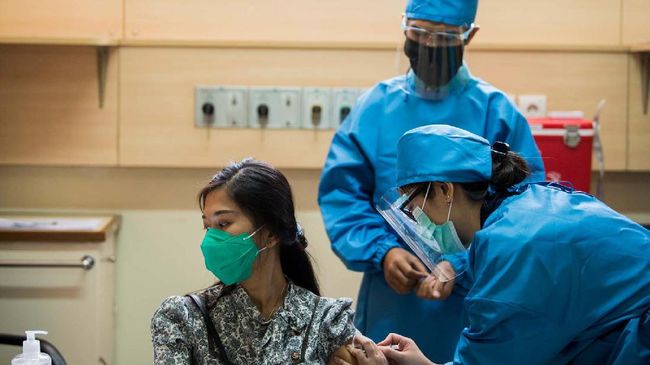
Jakarta, CNN Indonesia –
Head of the Covid-19 Vaccine Clinical Trial Research Team, Faculty of Medicine, Padjadjaran University (Unpad) Kusnandi Rusmil said, as many as 450 volunteers had received the injection of the vaccine made by Sinovac China. According to him, the clinical trial volunteers corona vaccine it does not indicate a health problem.
“Until now, there are 450 people who have been injected with the vaccine, 200 have wanted the second injection and 250 are just the first injection. Until now no one has been hospitalized or what, all just normal,” said Kusnandi when met at the Teaching Hospital (RSP). ), Unpad, Bandung, Wednesday (9/9).
According to Kusnandi, the reaction caused by the vaccine to volunteers was the same as immunization in general. At the beginning of the injection, for example, there is pain at the injection site for a while. However, the pain will then disappear quickly.
“At best, the complaints are a little fever, then the pain disappears within two days. It’s just like we are injecting vaccines at the Puskesmas. If someone has a fever, they are given paracetamol,” he said.
Kusnandi explained that every volunteer who is injected with the Covid-19 vaccine will see their progress. One of them is to check whether the volunteers have allergies or not.
“We always follow it. After the injection, the first 30 minutes we see whether there is an allergy or not. Then there is swelling or not. Then when we come home we are contacted again using the phone for two days in a row asked how good it is. Then in 14 days they come for the injection. second, “he said.
As is known, the implementation of vaccine clinical trials in Bandung involved 1,620 volunteers ranging in age from 18 to 59 years. Vaccine injections were carried out in six locations, namely Unpad Hospital, Dipatiukur Unpad Health Center, Garuda Health Center, Sukapakir Health Center, Dago Health Center, and Ciumbuleuit Community Health Center.
In addition to being domiciled in Bandung, prospective volunteers must also pass verification of physical health and are not exposed to Covid-19.
The injection of the Covid-19 vaccine clinical trials among volunteers was carried out randomly and confidentially. Some are given a vaccine or a placebo. It is not known whether they received the liquid vaccine under phase III clinical trials or just a placebo as a control device.
The clinical trial process for the Covid-19 vaccine will run for six months until the end of 2020. If it has been tested and has received distribution permits, China’s Sinovac vaccine will be mass produced in early 2021.
(hyg / DAL)
– .