Comirnaty, the vaccine from the German manufacturer BioNTech and its US partner Pfizer, has been in use in the United States with emergency approval since December. From now on, the vaccine can be injected as normal. The US Food and Drug Administration (FDA) has now granted the two companies’ application in May.
The approval is a “milestone” in the fight against the COVID-19 pandemic, said FDA Chief Executive Janet Woodcock: The public can be sure that the vaccine “meets the high standards of safety, effectiveness and manufacturing quality set by the FDA required by an approved product “. However, the regular approval is only valid for people over the age of 16 years. The emergency authorization for people aged twelve and over will continue to exist.
FDA chief Janet Woodcock: “A milestone” (archive)
–
In order for Comirnaty to get the green light, the FDA had to use an accelerated procedure. About ten times as much data was examined as for the emergency approval – including much that was gained from the massive use of the vaccine in the United States in the past few months.
Never before has the FDA had so much evidence to assess the safety of a vaccination in the testing process. BioNTech / Pfizer has administered more than 200 million doses since it was granted emergency approval in the United States. In the EU, the vaccine has so far received a one-year conditional marketing authorization.
New vaccination requirements expected
According to the expectations of many experts, full approval in the United States could result in further vaccination requirements – for example from US city administrations, universities or health facilities, which, for legal reasons, among other things, wanted to wait for full approval for such a step. The mayor of the metropolis of New York, Bill de Blasio, announced the compulsory vaccination for teachers and other staff in public schools. Defense Secretary Lloyd Austin had already promised compulsory vaccination for US soldiers.

Corona vaccination with BioNTech / Pfizer vaccine in Pasadena (California): More evidence of safety than ever before
–
Regardless of this, experts hope that the vaccination campaign will be boosted, because many people who have not yet been vaccinated stated in surveys that the reason for this was the lack of full approval. This could only have been given as a pretext. Nevertheless, the executive FDA boss Woodcock is confident that even more people will now be vaccinated against corona.
End of vaccination fatigue?
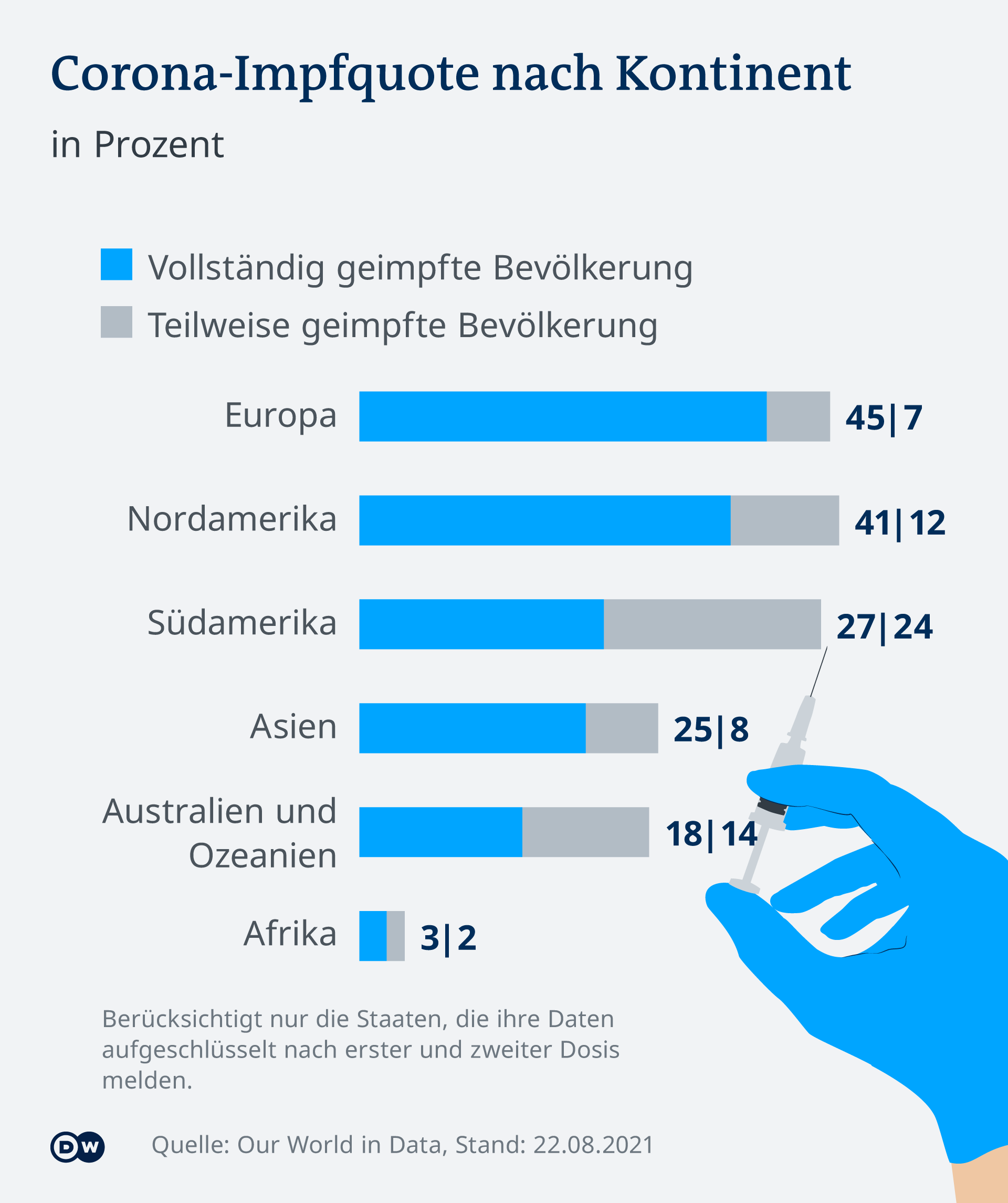
Vaccinations in the US had bottomed out in July. Since the delta variant ensures that more people infected with corona have to go to hospital again, the vaccinations are increasing again – one million doses per day were administered at the end of the week. Just over half the US population is now fully vaccinated with one of the three vaccines.
The vaccines from Moderna and Johnson & Johnson have also been used in the USA for months on the basis of emergency approvals. Moderna applied for full approval from the FDA at the beginning of June, but has not yet submitted all the necessary documents. Johnson & Johnson plans to submit such an application later this year.
 –
–

