Posted ,
Lecture 3 min.
–
Long considered an orphan disease, Cloves syndrome saw the birth of its first drug treatment in the United States. A medical advance made possible by a French study.
–
In the movie Elephant ManDavid Lynch staged the story of the British Joseph Merrick, suffering from Proteus syndrome (and yes the “syndrome d’Elephant Man” is not his real name, no offense to some!), close to Cloves syndrome. These are rare diseases characterized by atypical growths and abnormalities in the blood vessels, lymphatic system and other tissues.
Long considered an orphan disease (therefore intractable), Cloves syndrome saw the birth of its first treatment in the United States. American researchers from the laboratory Novartis have developed a molecule, the Vijoice (alpelisib), taken orally, would treat patients with Cloves syndrome. The placing on the market has been authorized by the American agency Food and Drug Administration.
Cloves syndrome, an orphan disease
Cloves syndrome (Congenital Lipomatous Overgrowth, Vascular Malformation, Epidermal Naevi) is a genetic disease causing accelerated deformities of organs and limbs, vascular swellings, growths of fatty tissue, sometimes associated with more or less severe scoliosis.
This pathology is linked to mutations of a gene called PIK3CA, which develop during embryonic life. If the mutation appears early, the symptoms will be very severe. Its symptoms can be debilitating and lead to disruptions in daily activities. The disease is rather rare since its prevalence in the world is less than one case in a million.
It is partly for this reason that therapeutic innovations for Cloves syndrome are almost non-existent. Pathology is not always easy to diagnose in its minor forms. The High Authority for Health (HAS) has never validated the national diagnostic and care protocol (PNDS) offered to the healthcare professionals concerned.
Until today, the only treatment options for patients were surgical or interventional radiology procedures.
Vijoice: the glimmer of hope
No drug treatment had been found to cure Cloves syndrome before. The laboratory Novartisoffers one today: Vijoice (alpelisib), which can be taken orally to treat affected patients.
Vijoice (alpelisib) is an oral α-specific inhibitor of phosphatidylinositol-3-kinase (PI3K), an enzyme that treats rare overgrowth conditions caused by the effects of mutations in the PIK3CA gene.
Concretely, this molecule will specifically inhibit the gene responsible for the disease, PIK3CA, ie it will prevent its operation.
The marketing of Vijoice has been authorized by the American agency Food and Drug Administration (FDA). But its use has not yet been approved outside the United States.
To better understand the long-term efficacy and safety of alpelisib on syndromes such as Cloves, the laboratory Novartis is conducting additional clinical trials.
A French origin
The authorization of the American agency would not have been made possible without the results of the EPIK-P1 study, conducted by Professor Guillaume Canaud (Necker Hospital – Sick Children AP-HP / INEM Center for Molecular Medicine – Inserm / Imagine Institute / Paris Cité University) – Novartis promotion.
The retrospective study was conducted on 57 patients, including 44 hospitalized at the Necker Hospital – Children Sick AP-HP.
The study showed that patients treated with Vijoice experienced a reduction in the size of their lesions and growths and an improvement in signs and symptoms related to Cloves syndrome. Guillaume Canaud and his teams have even been congratulated by the National Institute of Health and Medical Research (Inserm) for their work.
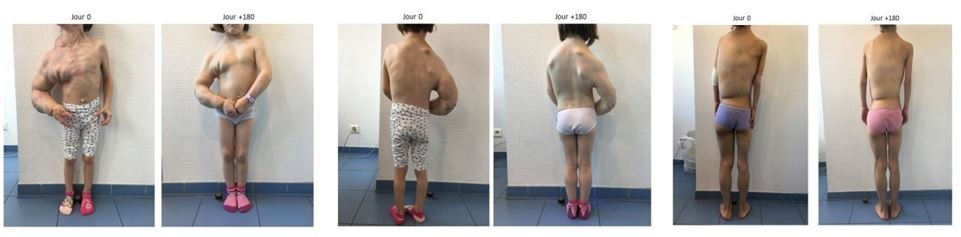
“I am proud of this exceptional achievement which will offer a possibility of drug treatment for patients suffering from overgrowth syndrome or vascular abnormalities linked to a PI3KCA mutation. This is the result of work for which multiple teams from the Necker Hospital – AP-HP sick children but also within the research laboratory (Institut Necker – AP-HP sick children – Paris Cité University) have worked. hand in hand with the laboratory that owns the molecule (Novartis), patient associations and the FDA”said Professor Guillaume Canaud.
“This is a major step forward in improving patient care”he concluded, offering hope for a better quality of life to affected patients and families.
–


