March 7, 2022
Credit: Disclosure
Pharmaceutical company Sanofi Medley announced that it will collect all batches of a Medley brand antihypertensive drug. The pharmaceutical announced the recall after noting the presence of mutagenic impurities in the products, which can pose a risk to the health of users.
The impurities, according to the company, could cause changes in the users’ DNA, increasing the possibility of cancer in the long term. However, as he points out, the specific risk of this chemical substance effectively causing cancer in humans is still unknown.
The products collected are:
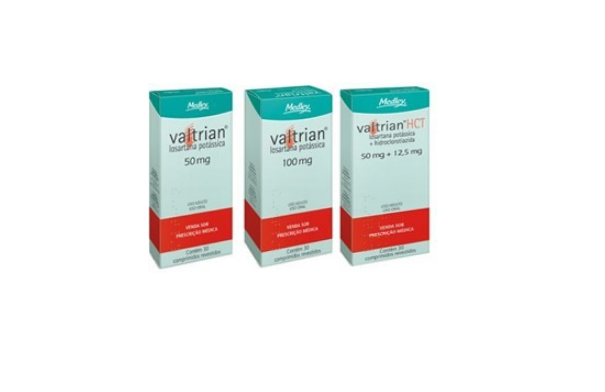
Losartan potassium + hydrochlorothiazide 50 mg + 12.5 mg;
Losartan potassium + hydrochlorothiazide 100 mg + 25 mg;
Losartan potassium 50 mg;
Losartan potassium 100 mg.
The recall is voluntary and the return process is free for consumers. However, it is important to consult your doctor to advise on the return and follow-up of the treatment.
Anyone who has any batch of these products, just call Medley’s SAC: 0800-703-0014.
Check out the statement in its entirety:
CNOTICE OF RECALL
Sao Paulo, February 10, 2022 — Sanofi Medley Farmacêutica Ltda informs the voluntary recall of all batches of products: Valtrian® HCT (Losartan Potassium + Hydrochlorothiazide) 50mg + 12.5mg, Valtrian® 50mg and 100mg (Losartan Potassium) Tablets.
This voluntary recall is being done by Sanofi Medley as a precautionary measure due to the presence of mutagenic impurities in the products.
A mutagenic impurity is a chemical substance that can cause a change in a cell’s DNA. These mutations may increase the long-term risk of cancer, but the specific risk of this impurity causing cancer in humans is currently unknown.
This collection does not represent a cost for patients. If you have any batch of these products, call Medley Customer Service at 0800-703-0014. In case of doubts about the treatment, the doctor should be consulted.
Regards,
Sanofi Medley Pharmaceuticals
–