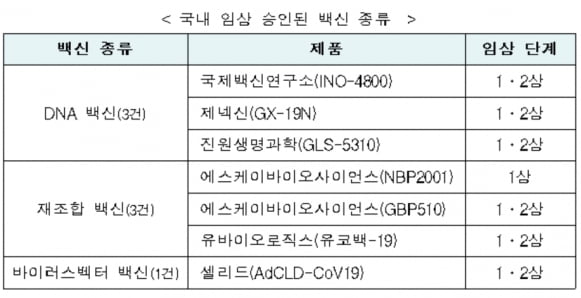
Ministry of Food and Drug Safety Ubiologics(24,500 -6.49%)Announced on the 21st that it has approved the clinical trial plan (IND) for the Corona 19 vaccine’Yukobac-19′ under development. Accordingly, the number of corona 19 vaccines in clinical trials in Korea has increased to seven.
According to the Ministry of Food and Drug Safety, this clinical trial aims to evaluate safety and immunogenicity in healthy adults. After the first phase, the second phase is sequentially performed.
UbiologicsYukobaek-19 is a recombinant vaccine made using genetic recombination technology of the surface antigen protein of the Corona 19 virus. The surface antigen protein of the vaccine stimulates immune cells to form neutralizing antibodies and induces an immune response. When the Corona 19 virus invades, antibodies remove it.
Yukobaek-19 uses liposomes as an immune booster. It is explained that the surface antigen protein is expressed on the surface of the liposome to induce an immune response.
Overseas, US NovaVax and others are conducting clinical trials of the Corona 19 vaccine using genetic recombination technology.
An official from the Ministry of Food and Drug Safety said, “We will do our best to ensure that our people can receive treatment opportunities by supporting the rapid development of safe and effective COVID-19 treatments and vaccines.”
–
Reporter Kim Ye-na [email protected]
–
Ⓒ Hankyung.com prohibits unauthorized reproduction and redistribution
–