Jakarta: Friend Medcom Do you know what is the lightest, simplest and most abundant chemical element in the universe? So true! Hydrogen. Element chemist this is the most abundant number found in the universe with a number that can reach 75 percent with an atomic form of hydrogen abundance reaching more than 90 percent. Well, how come?
Origins of hydrogen:
There are countless galaxies in the universe made up of stars that emit light. The light comes from the combustion of a fusion reaction where hydrogen is the fuel in the process.
This happens because several hydrogen nuclei combine and produce helium, a substance that produces positron particles and will later release energy that emits light from a star. Therefore, hydrogen is needed in very large quantities for stars to continue to emit their light.
Another unique fact about this content is that although it is widely distributed in spacebut in its dissolved state, hydrogen is the least chemical element on earth. They are usually found in the form of compounds, such as water and carbon. This happens because hydrogen gas is a very light gas, so even Earth’s gravity is unable to attract hydrogen molecules.
What do you think of this article?
After knowing the background, Medcom I would like to invite you to learn more about the physical and chemical properties of hydrogen. Curious? We see!
Physical and chemical properties of hydrogen:
Reported by ZenioThere are several physical and chemical properties of hydrogen that distinguish it from other chemical elements, including:
Physical properties:
- At normal temperatures, hydrogen is a gas (odorless, colorless and tasteless).
- It is the lightest gas
- Non-polar: a gas that does not dissolve in water
Chemical properties:
- It has a high binding energy
The table above shows how much energy is needed to separate hydrogen or H2 into two atoms (HH) because it’s very simple.
Inert gases and reducing gases
Due to its high binding energy, hydrogen has the property of an inert gas, a gas that is difficult to react. However, under high temperature conditions, this gas can be a reducing gas, a gas that reduces various elements and compounds.
It decomposes at very high temperatures
At high temperatures, which are more than 2,000 Kelvin or 1,726.85 ° C, they will dissociate the hydrogen molecules into smaller particles. The higher the temperature, the more hydrogen molecules will decompose, for example in the sun that no longer has H2 molecules because the average surface temperature has reached 5,778 Kelvin or 5,504.85 ° C.
The hydrogen atom has three ways to reach a stable state, namely:
1. Forms pure covalent bonds
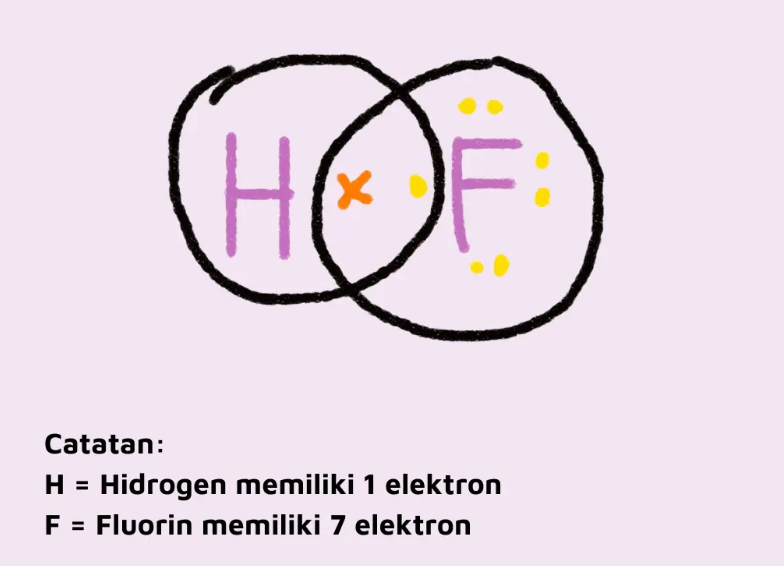
Hydrogen will form pure covalent bonds with other elements, for example hydrogen will form covalent bonds with fluorine (F) where hydrogen will take an electron of fluorine and vice versa. Hence, there is a simultaneous use of electrons so that both of them reach a stable state.
2. Release of electrons
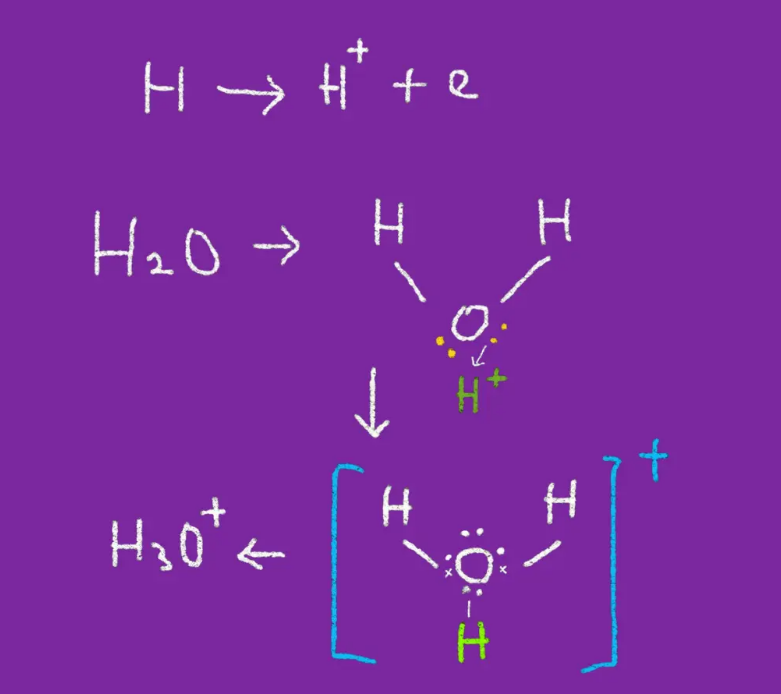
In a neutral state, hydrogen has only one electron which when it releases that electron, will form an H + ion which when coupled with a free electron, both will bond with each other and reach a stable state.
3. Capture the electrons
This can only happen if hydrogen combines with an electropositive element (metal), for example
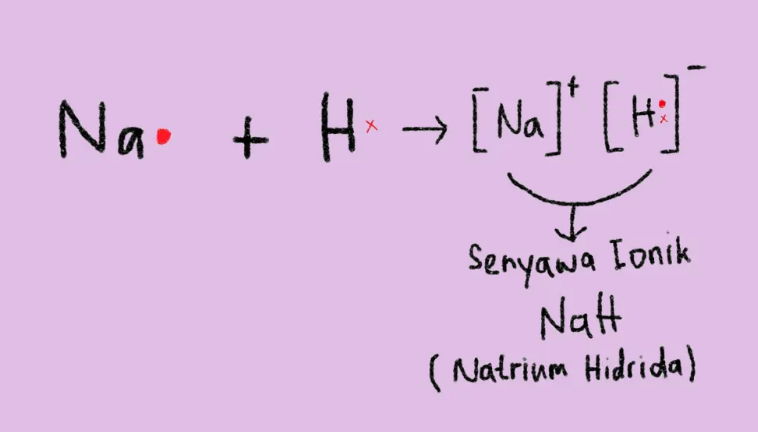
Sodium (Na) is a metallic element that is very easy to lose electrons. When the two elements combine, the electrons in sodium escape the hydrogen and the sodium will form positive ions, while the hydrogen will form negative ions (hybrids) which will subsequently form ionic compounds, namely NaH (sodium hydride).
Benefits of hydrogen
company Medcom Did you know that hydrogen has many benefits in various industries, you know! Between them:
- The urea fertilizer industry requires hydrogen as the raw material for ammonia
- The methanol industry uses hydrogen as a raw material
- The pulp, paper and textile industries use hydrogen peroxide as the main raw material for production and the bleaching agent
- The automotive industry is starting to use hydrogen as an environmentally friendly fuel for vehicles
- The pharmaceutical industry also involves hydrogen compounds in the manufacturing process of vitamins and other pharmaceutical products
- Hydrogen is also directly involved in heat treatment, annealing and sintering processes in the metallurgical industry.
- The mining industry requires hydrogen to remove the sulfur compounds contained in oil
- The margarine industry uses hydrogenation in the processing of oil or fat in margarine.
Well, that’s all about hydrogen. Hope it’s useful for you guys Medcom (Angelic grace)
(REN)


