Produced by SK Bio… Stable supply
NovaVax phase 3 clinical trial is not over yet… The timing of domestic use may become uncertain
○ NovaVax has high safety, but the effect is unknown
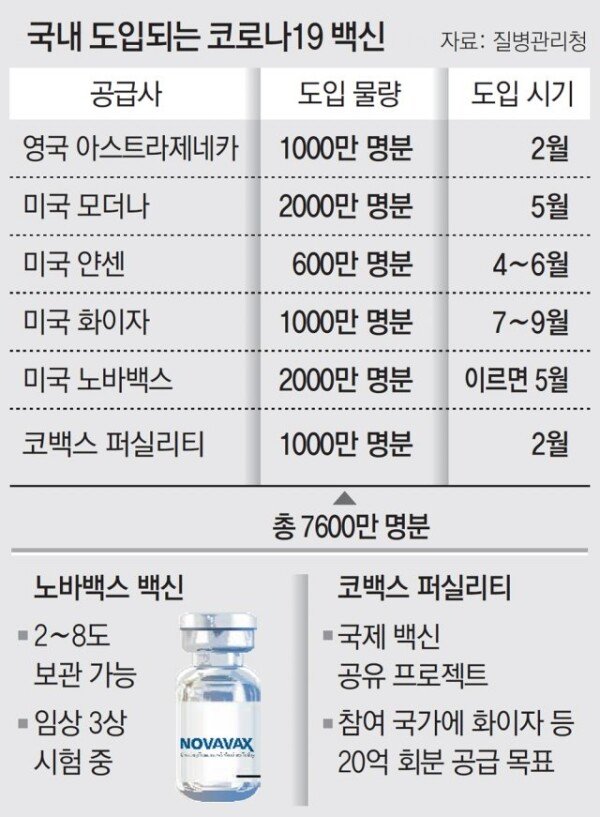
NovaVax is the first to transfer technology itself rather than importing finished vaccine products. Both AstraZeneca and Pfizer Janssen modders and vaccines, which have been confirmed to be introduced in Korea, are both imported from overseas or consigned to domestic production. Depending on the situation, supply and demand may be disrupted. However, stable supply is possible when domestic companies have the right to produce vaccines. President Moon Jae-in said, “Our people will be very pleased,” after a video call with Stanley Ark NovaVax CEO on the 20th. “The government (between NovaVax and SK Bioscience) will actively provide administrative support for production and supply. Will”. The NovaVax technology transfer agreement was finalized and the government’s vaccine introduction seems to have been virtually finished. A government official said, “Unless there are special circumstances, there are no plans to secure additional large-scale vaccines.”
NovaVax vaccine is evaluated to have high safety as there are already many precedents such as hepatitis B vaccine through protein recombination. It can be stored refrigerated (2-8 degrees), making it easy to distribute and inoculate. However, it is too early to predict the exact effect since the phase 3 clinical trial has not been completed. For this reason, it cannot be ruled out the possibility that the time of introduction in Korea will be delayed. Joong-sik Eom, a professor of infectious medicine at Gachon University Gil Hospital, said, “It is meaningful in terms of securing enough vaccines, but it is unclear how effective after vaccination, how long antibodies will last, and when the vaccine will be supplied. In fact, there have been reports from foreign media that NovaVax is having difficulties in clinical trials. According to the Washington Post (WP) in the United States, NovaVax recruited 30,000 clinical investigators from the United States from December last year, but only 9,000 participants until last week.
○ Pfizer’s strongest vaccination for the first time in Korea
The introduction of the Corona 19 vaccine through Cobax Facility is also speeding up. The vaccine to be introduced in Korea for the first time through COVAX was confirmed as a Pfizer vaccine. The government is in the middle of preparing for vaccination in line with the introduction of the Pfizer vaccine. A government official said, “Pfizer vaccines received through COVAX have been tested for quality by the World Health Organization (WHO). As experts from the Ministry of Food and Drug Safety (MFDS) also participated in the inspection, we will make it available immediately through the’special import’ procedure when introduced in Korea.” The special import procedure is a system that allows drugs that need urgent use to be used in Korea without going through the formal approval procedure. Remdesivir, currently used as a treatment for Corona 19, has also been approved for use in Korea through a special import procedure. As planned, Pfizer vaccination is expected to be available from early or mid-February. Initially, the quarantine authorities had revealed that if the UK AstraZeneca vaccine was officially approved by the Ministry of Food and Drug Safety in mid-February, it would be possible to start vaccination of priority targets from the end of February. When the Pfizer vaccine comes in, distribution and preparation for vaccination are even more important. MRNA vaccines such as Pfizer and Modena should be distributed and stored at a cryogenic temperature of -20°C to -70°C. Five people must be vaccinated at one time, and if the number of people is insufficient, the remaining amount is discarded. An official from the Korea Centers for Disease Control and Prevention said, “We expect that the exact amount and timing of the vaccine (Pfizer) that will be introduced through COVAX will be determined at the end of January or after.” We are preparing the details.”
Image [email protected]Go to reporter page>Reporter Kim So-young and Lee Eun-taek
Copyright by dongA.com All rights reserved.
—
–