You can now follow the latest news for free through our Facebook page
Click here to subscribe
—
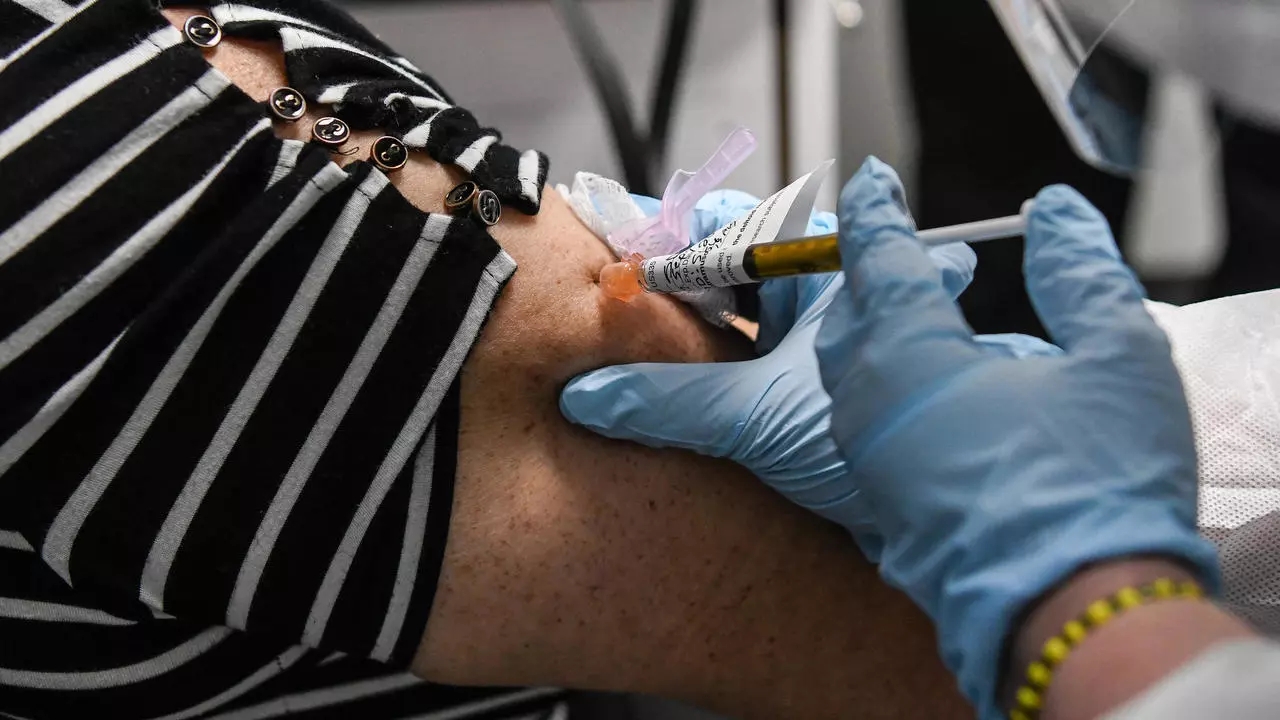
Washington: Novavax, the American biotechnology company, announced Thursday that it has begun the third phase of clinical trials in Britain for its vaccine against Covid-19, making it the eleventh experimental vaccine in the world that reaches this final stage.
The company said that 10,000 volunteers between the ages of 18 and 84 will participate in these experiences.
“Because of the current high level of SARS-Cove-2 transmission, and given that this level will likely continue to be high in the UK, we are optimistic that this is the third phase of the trials,” the statement quoted Gregory Glenn, the company’s director of research and development. The pivotal clinical will benefit from a rapid volunteering process and a short-term evaluation of the vaccine’s effectiveness will be provided.
This is the eleventh vaccine in the world that enters the final stage of clinical trials, in which tens of thousands of volunteers are participating. They are usually divided into two equal parts: half receives a placebo vaccine and the other half receives the experimental vaccine.
The most advanced western project on the path to producing a deadly virus vaccine is the experimental vaccine developed by Oxford partner AstraZeneca, and two other vaccines developed by Pfizer and Moderna.
In parallel, similar projects are underway in China and Russia.
Novavax is one of six companies that received hundreds of millions of dollars in funding from the US federal government to produce a vaccine against the emerging corona virus, and it is the fifth whose experimental vaccine enters the third phase.
The company has raised more than $ 1.6 billion in US public funds to produce 100 million doses of the vaccine.
– .