ENGINEERINGNET.BE – When a virus invades, so-called first responders let other cells in their environment know that they are infected. These first responders are the first to produce signaling proteins known as type I interferons, or IFN-Is.
This kickstarts the production of IFN-Is in other cells, including immune cells. “That creates a kind of domino effect,” says PhD student Laura van Eyndhoven.
“But if too much IFN-Is is produced, it increases the chance that someone will develop an autoimmune disease. If too little of it is produced, it can lead to unsuccessful clearing of viruses.”
In the case of Covid-19, this can cause cytokine storms, uncontrolled inflammation leading to serious life-threatening conditions, and chronic inflammation.
Van Eyndhoven: “For my research I focused on experiments with primary immune cells, isolated from healthy donors and from patients with autoimmune diseases.”
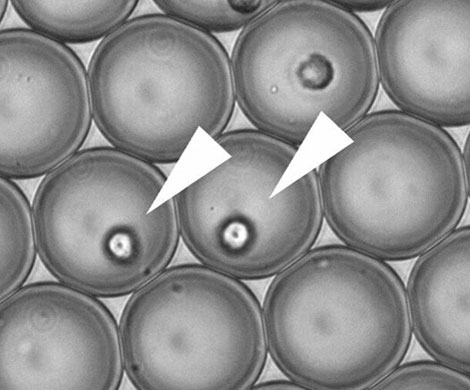
To study individual immune cells in the lab, Van Eyndhoven used microfluidic devices. “I was able to study and activate individual immune cells in picolitre droplets, which was virtually impossible in the past. This provided insight into how an individual cell communicates with cells that may be nearby.”
By combining microfluidics with computer models, Van Eyndhoven was able to study interferon production in individual cells and compare the results with those of large groups of cells.
She and her colleagues made a startling discovery about the fate of first responder cells that could serve as an important finding when it comes to improving IFN-Is targeted therapies.
“Only between 1% and 3% of cells are first responder cells. However, these cells do not randomly start making IFN-Is upon infection, as was previously believed. My research shows that these cells are predisposed to first to be responders.”
The insights provided by Van Eyndhoven’s research are a stepping stone to understanding the fundamentals of how individual cells collectively perform systemic functions and how they can be manipulated for therapeutic treatments.
“I envision a future where personalized therapies target aberrant cytokine secretion profiles, as seen in numerous autoimmune diseases,” said Van Eyndhoven.