Natural gas, consisting mainly of methane, has a relatively low energy density under ambient conditions. The partial oxidation of methane to methanol increases energy density and stimulates the production of many chemicals. An international research team led by scientists at University of Manchesterdeveloped a fast and economical method for converting methane or natural gas into liquid methanol at ambient temperature and pressure.
The scientists used visible light to drive the conversion under a continuous flow over the photocatalytic material. Using neutron scattering from the VISION instrument, they observed how the process works and how selective it is.
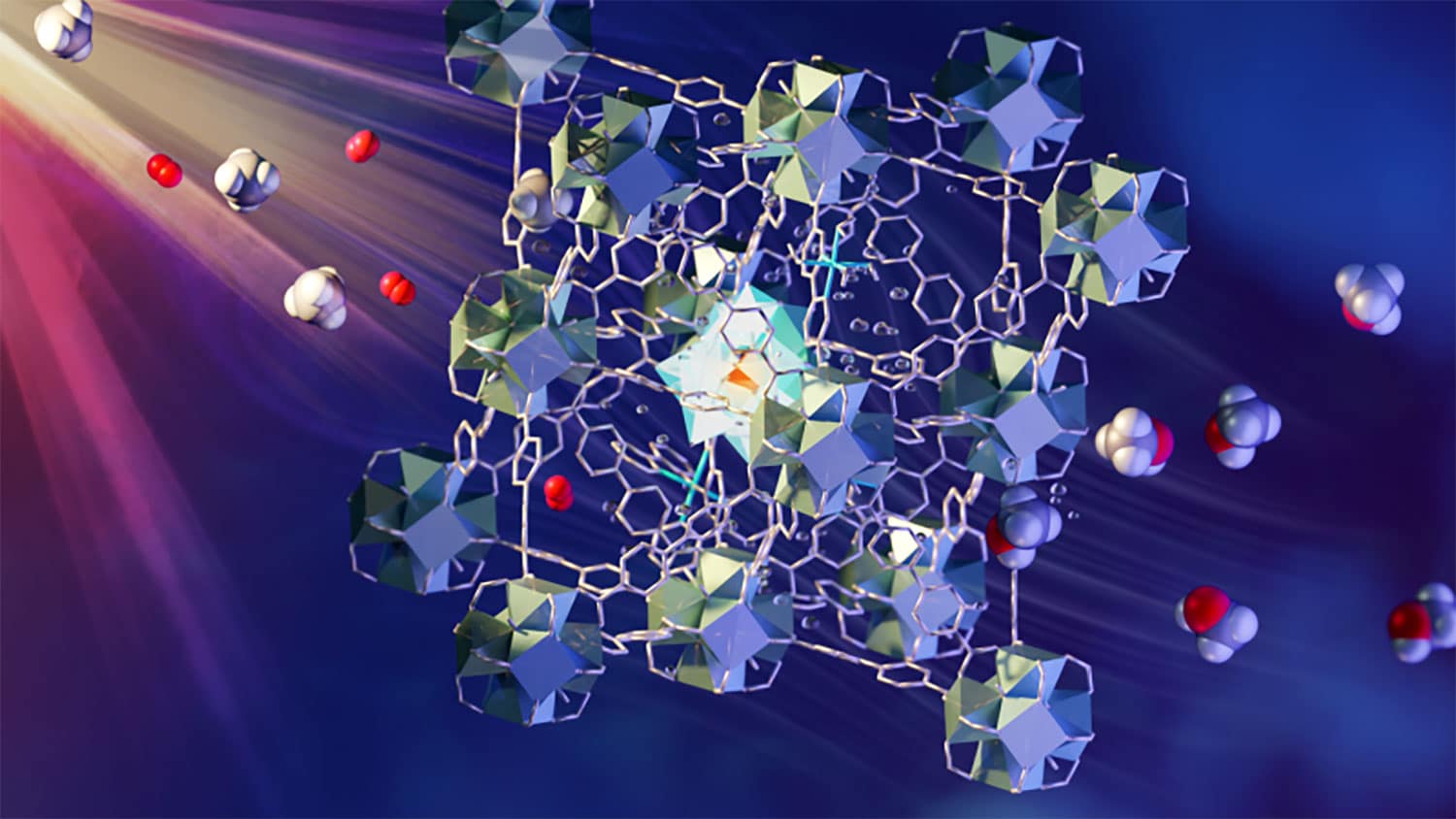
This method involves a continuous flow of saturated methane/oxygen water over a new MOF catalyst. Various components in MOF have roles in absorption lampElectron transfer, activation and assembly methane and oxygen. Liquid methanol is easily extracted from water. This process is generally regarded as the “holy grail of catalysis”.
The difficulty of weakening or breaking the chemical bonds between carbon and hydrogen (CH) to include oxygen atoms (O) forming C-OH bonds is the main obstacle in converting methane (CH4) into methanol (CH3OH). Steam reforming and synthesis gas oxidation are usually two conventional stages methane conversion processwhich require high temperatures and pressures and are energy-intensive, expensive, and inefficient.
This newly developed process is fast and economical. It uses multi-component MOF and visible light to drive the conversion process. When exposed to light, a layer of MOF grains is passed through a stream of water saturated with methane and oxygen. The metal-organic framework consists of various designed elements which are stably housed within a porous superstructure. Together, they absorb light to form electrons, which are then transferred to oxygen and methane in the pores to form methanol.
Sihai Yang, a professor of chemistry at Manchester and a correspondent author, said, To greatly simplify the process when methane is exposed to a functional MOF containing iron monohydroxy sites, the activated oxygen molecules and energy from light activate the CH bonds in methane to form methanol. This process is 100% selective – meaning no unwanted by-products – it can be compared with methane monooxygenase, which is the enzyme in nature for this process.”
Investigations show no loss of performance when the solid catalyst is isolated, cleaned, dried and reused for at least ten cycles, or about 200 hours of reaction time.
The new photocatalytic method can be compared to how plants use photosynthesis to convert light energy into chemical energy. Through its leaves, the plant takes carbon dioxide and sunshine. These substances are then converted into sugar, oxygen and water vapor through photocatalysis.
Martin Schroeder, Vice President and Dean of the School of Science and Engineering at Manchester and related author, said, This process has been called the “holy grail of catalysis.” Instead of burning methane, it is possible to convert the gas directly into methanol. These high-value chemicals can be used to produce biofuels, solvents, pesticides, and fuel additives for vehicles. This new MOF material can also facilitate other types of chemical reactions by serving as a kind of test tube where we can combine different materials to see how they react. “
Yongqiang Cheng, instrumentation scientist with ORNL’s Directorate of Neutron Science, said: “Using neutron scattering to capture the ‘image’ with the VISION instrument initially confirmed a strong interaction between CH4 and the iron-hydroxyl monomer site in the MOF that weakens the CH bond.”
Anibal “Timi” Ramirez Cuesta, who leads the Chemical Spectroscopy Group at SNS, He saidDan VISION is a high-throughput neutron spectrophotometer that has been optimized to provide information about molecular structure, chemical bonds, and intermolecular interactions. The methane molecule produces a strong and characteristic neutron scattering signal from its spin and vibration, which is also sensitive to the local environment. This allowed us to clearly detect the bond-weakening interactions between CH4 and MOF using advanced neutron spectroscopy techniques.”
The new conversion method can significantly reduce equipment and operating costs by eliminating the need for high temperatures or pressures and using energy from sunlight to drive the photo-oxidation process. The high processing speed and ability to convert methane to methanol without unwanted by-products will facilitate the development of a cost-effective direct treatment.
Journal reference:
- An, B., Li, Z., Wang, Z. et al. Direct photooxidation of methane to methanol via the monohydroxy iron sites. grout. rain. (2022). DOI: 10.1038 / s41563-022-01279-1
–