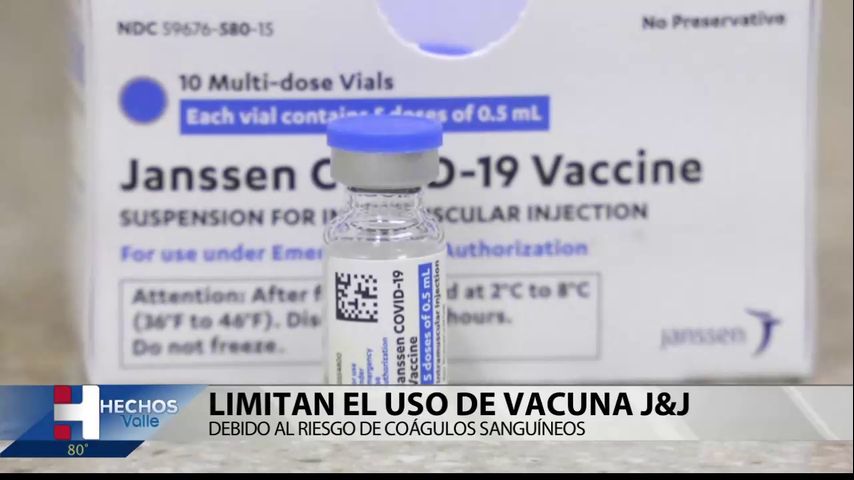
The FDA announced that it will limit the emergency use authorization of Johnson & Johnson’s COVID-19 vaccine to people 18 years of age and older.
In an FDA statement, it was reported that the change is motivated by the risk of a rare and dangerous clotting condition called thrombosis with thrombocytopenia syndrome, which can occur after receiving the vaccine.
The agency confirmed that the updated authorization also applies to booster doses.
–
–
Related posts:
The fourth leading cause of death is inactivity! 70% of Chinese people are as immobile as a mountain...
The "100 Days of Health" campaign provides more than 129 million free services within 82 days
only 27% of adolescents receive the third dose in Río Negro
Swollen Tonsils: Causes, Symptoms, and Treatment
/data/photo/2022/05/07/62766f677e3cf.jpg)