..Drafting.
The Superior Council of Scientific Investigations (CSIC) and Biofabric have decided to withdraw the application before the Spanish Medicines Agency (Aemps) to carry out a clinical trial of its vaccine against Covid-19, “before the difficulties to complete it in Spaingiven the very high percentage of the vaccinated population and the incidence of the new Omicron variant».
The CSIC and Biofabri withdraw the request to test the Mariano Esteban vaccine “due to the difficulties in completing it in Spain” due to the high vaccination coverage
In any case, CSIC and Biofabri have decided that research on this vaccine, led by researchers Mariano Esteban and Juan Garcia Arriaza at the National Center for Biotechnology (CNB-CSIC), “continues to adapt its vaccine model to new variants”.
“After analyzing the current vaccination situation against SARS-CoV-2 virus infection in Spain, we have assessed the window of opportunity to carry out this clinical trial, and we have decided to withdraw the evaluation dossier that we have presented to the AEMPS«as confirmed by CSIC sources to Europa Pressafter the information advanced by Medical Writing.
In August 2021, the AEMPS requested a series of “clarifications” about some specific aspects of the clinical trial to the researchers. “It is a routine procedure, it is quite routine that this type of clarification is requested in any trial. The current status is that clarifications have been requested on a number of issues. We will be waiting for these clarifications to occur”commented at the time Minister of Health, Carolina Darias.
The CSIC has reported that the research directed by Mariano Esteban on this vaccine “continues to adapt its vaccine model to new variants”
At the time, it was rumored that the human clinical trial was not authorized because the vaccine caused lung damage in a monkey. But the CSIC denied that the paralysis of the process was due to the death of this animal.
The Science Minister Diana Morantexplained in January why the AEMPS did not give its approval to human trials of the vaccine. “The explanation is that science is like that, it has its processes. Research does not always achieve the results one would like”detailed the minister at an informative breakfast organized by Europa Press last January 31.
The phase I trial, for which there was going to be about a hundred volunteers, it was going to take place at the Hospital de la Paz. This hospital had already started recruiting. In phase I, 112 volunteers were going to participate and the effect of the doses would be analyzed, while in phase II the immunogenicity and safety results would be evaluated in 500 volunteers. Finally, in phase III, between 20,000 and 30,000 healthy people were going to be recruited to evaluate the efficacy of the vaccine.
“Science is like that, it has its processes. Research does not always achieve the results one would like”
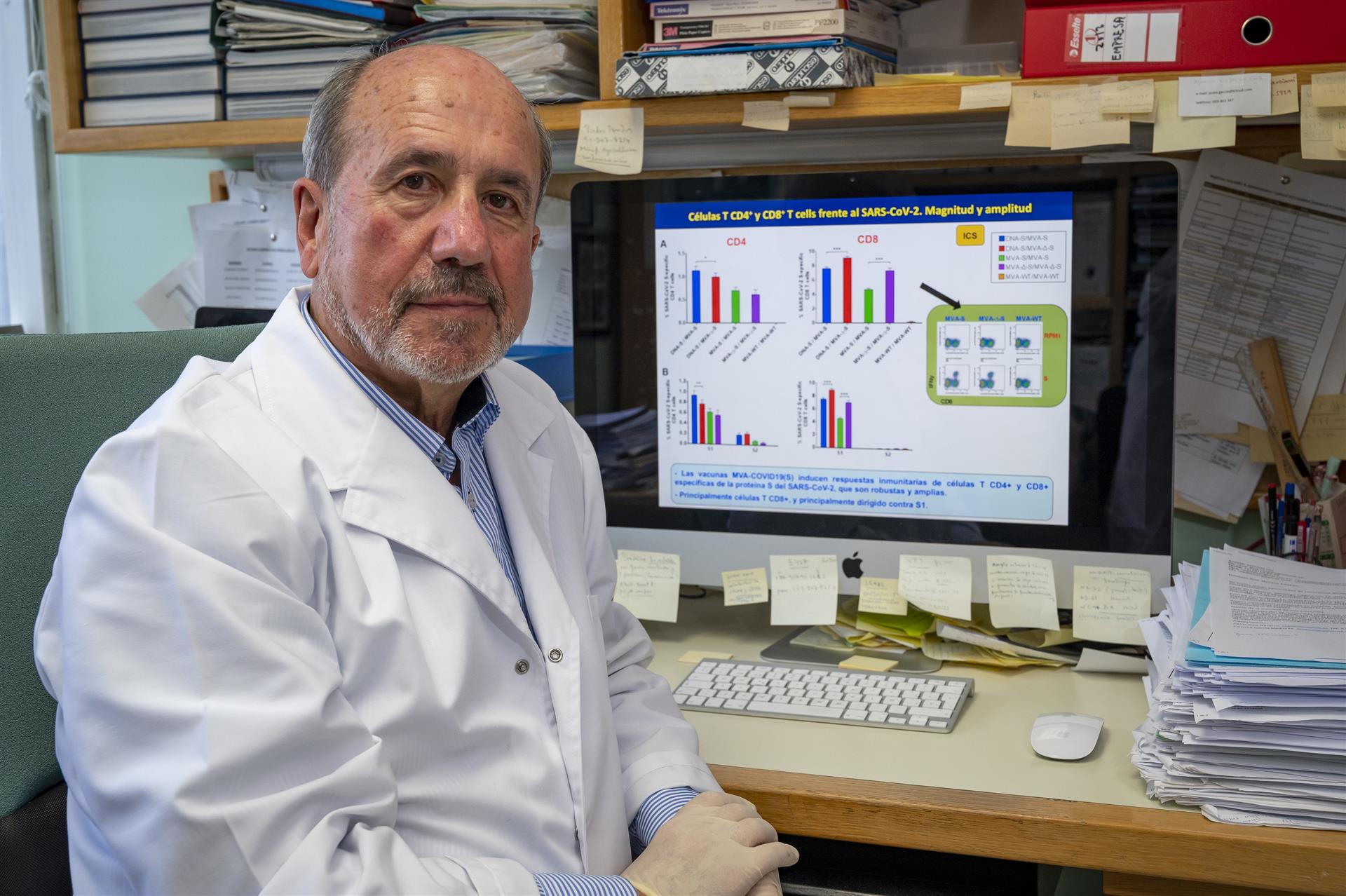
Esteban and Arriaza’s project consisted of using as a vaccine vehicle a highly attenuated smallpox family virus to introduce the spike protein of SARS-CoV-2, which is what allows the virus to enter cells. In this way, it would be possible to immunize people so that they generate antibodies against that protein.
In any case, the minister congratulated Esteban and “all the lines of research that fought and contributed to knowing more about the virus.” “Science has not been just about vaccines, it has given many scientific results that we have taken advantage of to live with the virus”concrete.
Complementary news
–