Pixabay
–
For the first time, scientists have managed to capture the ‘quantum attraction’ between adjacent water molecules.
–
Nationalgeographic.co.id – For the first time, scientists captured the “quantum attraction” between water molecules adjacent. The research highlights the network of hydrogen bonds that give water its peculiar properties, which play an important role in many chemical and biological processes.
A team of scientists consisting of researchers from the Department of Energy’s SLAC National Accelerator Laboratory, Stanford University and Stockholm University in Sweden made direct observations with sunlight. laser. The goal is to find out how the hydrogen atoms in water molecules pull and push water molecules neighbors when excited.
It is well known that water is the most abundant but least understood liquid in nature. It shows a lot of strange behavior that is still difficult to explain by scientists.
Most liquids become denser as they get colder, water being very dense at 39 degrees Fahrenheit, just above its freezing point. This is why ice floats to the top of drinking glasses and lakes freeze from the surface down, allowing marine life to survive the winter.
Water also has an unusually high surface tension, which allows insects to walk on its surface. Water also has a large capacity to store heat, keeping ocean temperatures steady.
And this study seeks to reveal effects that could support key aspects of the microscopic origin of water’s peculiar properties and could lead to a better understanding of how water helps proteins function in living organisms. The results of the study have been published in Jurnal Nature on August 25, 2021.
Also Read: If a fish is thirsty, does it drink seawater?

Dawn Harmer/SLAC National Accelerator Laboratory
–
Researchers at the Dawn Harmer/SLAC National Accelerator Laboratory.
–
“The question is whether this quantum effect could be the missing link in the theoretical model describing the anomalous nature of water,” he added.
Each water molecule contains one oxygen atom and two hydrogen atoms, and the network of hydrogen bonds between the positively charged hydrogen atoms in one molecule and the negatively charged oxygen atoms in the neighboring molecule holds them all together. This complex network is the driving force behind many of the unexplained properties of water, but until recently, researchers were unable to directly observe how water molecules interact with their neighbors.
Meanwhile, collaborator Kelly Gaffney, a scientist at the Stanford Pulse Institute at SLAC said that this study is the first to directly show that the response of the hydrogen bond network to energy impulses is highly dependent on the quantum mechanical properties of how the hydrogen atoms are placed.
Also Read: Recent Research, There is Abundant Water in the Crust of Planet Ceres
It has long been suggested that this is responsible for the unique attributes of water and its hydrogen bond network. “The low mass of hydrogen atoms accentuates their quantum wave-like behavior,” he said.
How is Research Conducted?
Until recently, making these observations was a challenge because the hydrogen bonding motion was so small and fast. This experiment overcomes that problem by using the MeV-UED SLAC, a high-speed “electron camera” that detects the motion of fine molecules by scattering a strong electron beam from the sample.
The research team created a jet of liquid water 100 nanometers thick, about 1,000 times thinner than a human hair, and set the water molecules to vibrate with an infrared laser beam. Researchers then exploded the molecule with a short boost of high-energy electrons from the MeV-UED. It produces a high-resolution portrait of the molecular shift atomic structure assembled into a film stop-motion about how networks of water molecules respond to light.
Also Read: If a fish is thirsty, does it drink seawater?
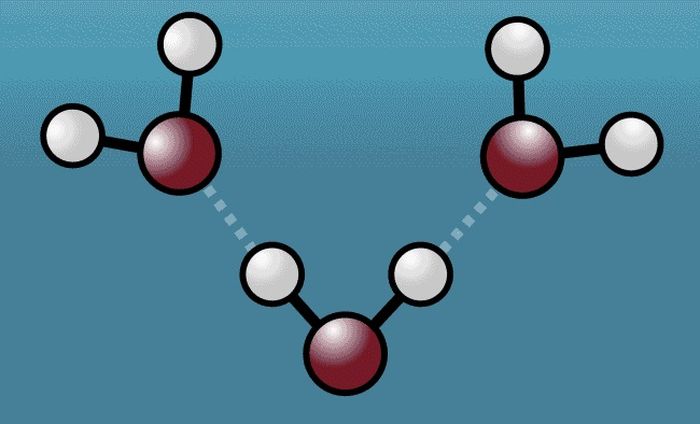
Greg Stewart/SLAC National Accelerator Laboratory
–
When the excited water molecules begin to vibrate, the hydrogen atoms (white) pull the oxygen atoms (red) from the closer neighboring water molecules, before pushing them away, expanding the intermolecular space.
–
From the captured footage, scientists revealed that when an excited water molecule begins to vibrate, its hydrogen atom pulls the oxygen atom of the neighboring water molecule closer together before pushing it away with its newfound force, expanding the space between the molecules.
“For a long time, researchers have been trying to understand hydrogen bond networks using spectroscopic techniques. The beauty of this experiment is that for the first time we can directly observe how these molecules move,” said Jie Yang, a former SLAC scientist and now a professor at Tsinghua University in China.
The researchers hope to use this method to gain more insight into the quantum properties of hydrogen bonds and the role they play in the peculiar properties of water, as well as the key role these properties play in many chemical and biological processes.
“Now that we can finally see hydrogen bonds move, we want to link that motion to the broader picture, which can explain how water led to the origin and survival of life on Earth and inform the development of renewable energy methods,” said Xijie Wang, staff scientist and SLAC’s leading study collaborator.
Also Read: Water Is Life, How Big Are Our Groundwater Reserves?
PROMOTED CONTENT
Featured Videos
– .

:quality(80)/cdn-kiosk-api.telegraaf.nl/d30252c0-0f1a-11ec-9d55-02d2fb1aa1d7.jpg)
