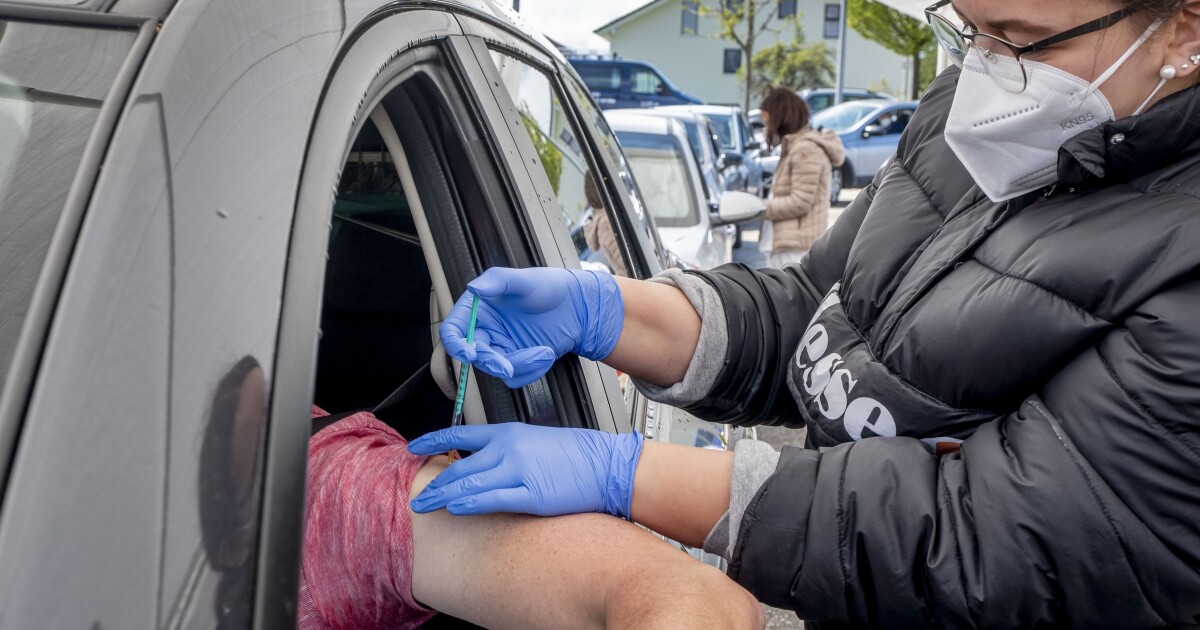
BERLIN (AP) – Germany authorized AstraZeneca’s coronavirus vaccine for all adults on Thursday in a bid to immunize as many people as possible as quickly as possible, Health Minister Jens Spahn announced.
Millions of European citizens have received the injection of AstraZeneca but concerns remain about a very small number of cases of blood clots. This has led some people in Germany to refuse to inject AstraZeneca in hopes of getting another one.
Spahn said that many people in low-risk population groups would be happy to receive the AstraZeneca injection. That is why the government decided to allow medical offices to offer this vaccine to all adults.
“It is very important to be pragmatic and flexible,” said the minister.
He suggested that some citizens do not want to get the AstraZeneca vaccine due to the long waiting period between the two doses: 12 weeks, compared to half that for Pfizer-BioNTech.
AstraZeneca now says that the second dose can be applied between four and 12 weeks after the first. Spahn affirmed that the government will leave “in the hands of the doctors” the decision of how long to wait between one dose and another.
The European Union drug regulatory agency has approved four coronavirus vaccines: AstraZeneca, Moderna and Pfizer, which require two injections each, and Johnson & Johnson, which requires only one application.
Germany’s vaccination campaign was initially delayed but has improved to the point that 15 million doses were given in April, the equivalent of the previous three months combined, Spahn said.
On April 28, Germany applied more than 1 million doses in a single day for the first time.
Germany has suffered more than 84,000 deaths from coronavirus since the start of the pandemic.
–