Infectious disease specialist at Robert Schuman hospitals, Dr Gerard Schockmel responded Tuesday evening to questions from the public about the precious serum developed against covid-19. The editorial team offers you a summary of the answers to the eight most frequently asked questions.
Infectious disease specialist at Robert Schuman hospitals, Dr Gerard Schockmel responded Tuesday evening to questions from the public about the precious serum developed against covid-19. The editorial team offers you a summary of the answers to the eight most frequently asked questions.
–
What are the different types of vaccines possible?
“There are three main families of vaccines: live attenuated vaccines, inactivated vaccines and subunit vaccines. The first contains live pathogens, but whose virulence has been attenuated, the second uses inactivated pathogens. In the third variant, we do not take the virus itself, but only the viral protein.
In other words, the body is trained to fight the virus. This is particularly the case with vaccines currently being developed against covid-19. This uses a small component of the virus’s RNA [un composé chimique essentiel dans le transport du message génétique et la synthèse des protéines, ndlr] that enters the cells.
Dr Gérard Schockmel has spent ten years researching RNA viruses.
Photo: Pierre Matgé/archive
–
–
How an RNA vaccine works ?
“Messenger RNA transmits information to human cells, which then use it to develop so-called ‘spike’ proteins. It is these which then activate the immune response, in other words the development of antibodies. However, these molecules are very fragile and break down quickly. In other words, they do not enter the cell nucleus and therefore do not alter the genetic material of the vaccinated person.
Why is it used here?
“Several reasons explain the choice of this type of vaccine. First of all, because it is relatively easy to develop. The Moderna laboratory was thus able to start designing its vaccine just two days after the virus was sequenced. [action consistant à analyser l’ADN des cellules et permettant ainsi de savoir de quels gènes elles sont composées, ndlr]. Also, compared to other vaccines, only very small doses of the substance are needed to have an effect. The immune response, that is, the development of antibodies, is also stronger than in people who have naturally acquired immunity to the disease.
360 videos are not supported. Watch the 360 video in the Youtube app.
–
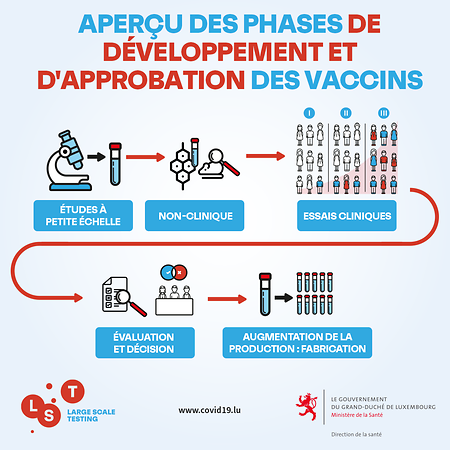
What tests are done on a vaccine before it is approved?
“The first tests are carried out on animals, because tests on human volunteers are not authorized. Different animals are then exposed to the vaccine, such as rats, mice or macaques. There are then three phases of clinical trials, each expanding the scope of testing. For example, Phase 3 of the Pfizer-BioNTech vaccine involved 44,000 people, half of whom received an injection.
–
All of these clinical phases have been completed at the pace required as part of this rapid vaccine development process. It is the time intervals between each clinical phase that have been shortened and allowed for overlap. More concretely, regulatory agencies, such as the European Medicines Agency (EMA) were informed of the progress of trials during clinical phases rather than in a final report after their completion, as is usually the case. . The results were reviewed by independent external experts.
Will the mutation of the virus from the UK affect the effectiveness of the vaccine?
Fortunately, the immune system is mobilized against the spike protein in general. As a result, the body develops different forms of antibodies that are effective against different parts of this protein. So even a mutated virus cannot easily overcome this protection. Currently, specialists assume that the protection of the vaccine will not be affected by the new variant of the virus, they are not very worried about this.

 –
–
 –
–
 –
–
 –
–
 –
–
 –
–
