Ministry of Food and Drug Safety, a dedicated approval review team
(Seoul = Yonhap News) Reporter Gye Seung-hyun = The Ministry of Food and Drug Safety explained the approval process of a vaccine and treatment for a novel coronavirus infection (Corona 19), which is recently being developed at home and abroad.
Foreign pharmaceutical companies AstraZeneca, Janssen (Johnson & Johnson), and Pfizer’s vaccines are currently undergoing preliminary review by the Ministry of Food and Drug Safety.
Celltrion, a domestic antibody treatment development company, said it plans to apply for use approval during this month.
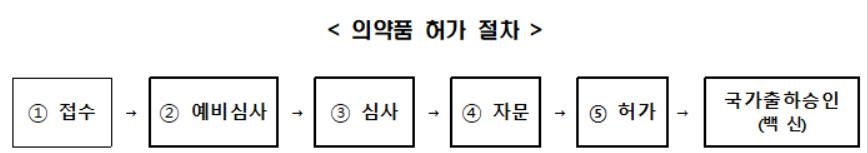
The drug approval process begins when a manufacturer or importer applies for product approval. When applying for a license, a company must submit data on non-clinical, clinical, quality, etc. necessary for the permission stipulated in Articles 31 and 42 of the Pharmaceutical Affairs Act to the Ministry of Food and Drug Safety.
–
[식품의약품안전처 제공. 재판매 및 DB 금지]
–
The Ministry of Food and Drug Safety aims to shorten the existing processing period, which is 180 days, and process it within 40 days through a preliminary review of each item and a prompt review by a dedicated permit review team.
Vaccines, which are biological products, can be distributed and sold only after passing the national shipment approval, which checks the quality once more.
In the case of the Corona 19 vaccine, we plan to shorten the processing period for the existing national shipment approval, which is usually 2 to 3 months or longer, with the goal of processing within 20 days.
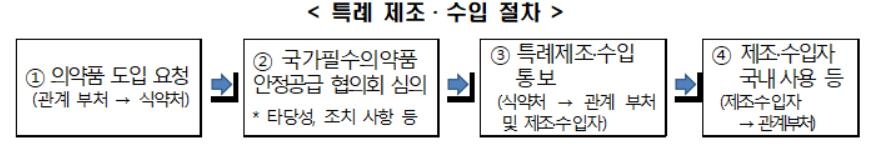
Pharmaceuticals can be manufactured or imported only after obtaining product permission, but Article 85-2 of the Pharmaceutical Affairs Act provides for special approval procedures.
The head of related ministries such as the Korea Centers for Disease Control and Prevention may request special manufacturing or import from the head of the Ministry of Food and Drug Safety to cope with infectious diseases, etc. If this is approved, it is possible to manufacture or import drugs that have not been approved for product in Korea.
–
[식품의약품안전처 제공. 재판매 및 DB 금지]
–
The vaccine is being tested in phase 3 clinical trials at a number of companies around the world.
As of the 25th of this month, Pfizer vaccine has been approved for emergency use in eight countries, including the United States and the United Kingdom, and conditionally licensed in the European Union and Switzerland. Modena vaccine was approved for emergency use in the United States.
In Korea, five products are currently in clinical trials, and most are in the initial phase, phase 1 or phase 1/2.
In the case of treatment, multinational pharmaceutical companies Lily and Regeneron are undergoing phase 3 clinical trials of antibody treatments, and they have received approval for emergency use in the United States.
Clinical trials are also being conducted to add the efficacy of COVID-19 treatment to existing drugs such as’varicitinib’, an ingredient for treating arthritis.
In Korea, a total of 15 products (13 ingredients) are in clinical trials, including antibody treatments that are being developed as a new COVID-19 treatment.
[표1] Corona 19 vaccine clinical trial progress
| Serial number | Requester | product name | Clinical trial contents (summary) | step | Approval date |
| 1 | International Vaccine Research Institute | INO-4800 | Evaluation of the safety, tolerability and immunogenicity of the COVID-19 vaccine using electroporation (EP) after intradermal vaccination in healthy adults | 1/2a phase | 2020-06-02 |
| 2 | SK Bio Science Co., Ltd. |
NBP2001 | Evaluation of the safety, tolerability, and immunogenicity of the COVID-19 vaccine in healthy adults aged 19 to 55 | 1 phase | 2020-11-23 |
| 3 | Sellide Co., Ltd. | AdCLD-CoV19 | Confirmation of the safety and immunogenicity of the COVID-19 vaccine for healthy adult volunteers | 1/2a phase | 2020-12-04 |
| 4 | Jinwon Life Science Co., Ltd. | GLS-5310 | Evaluation of the safety, tolerability and immunogenicity of COVID-19 vaccine administered intradermally to healthy adults | 1/2a phase | 2020-12-04 |
| 5 | Genexine | GX-19N | Exploring the safety, tolerability, and immunogenicity of the COVID-19 vaccine in healthy adults | 1/2a phase | 2020-12-11 |
[표2] Current status of clinical trials for COVID-19 treatment
| Serial number | Requester | product name (Ingredient name) |
Clinical trial contents (summary) | step | Approval date |
| 1 | Bukwang Pharmaceutical Co., Ltd. | Levovir Capsule 30mg (Clevudine) |
Evaluation of the safety and effectiveness of commercially available drugs (hepatitis B drugs) for patients with moderate corona19 | 2 phase | 2020-04-14 |
| 2 | NG Chem biology |
EC-18 | Evaluation of the safety and effectiveness of clinical trial drugs (neutropenia drugs) for patients with COVID-19 pneumonia | 2 phase | 2020-05-12 |
| 3 | Shinpoong Pharmaceutical Co., Ltd. | Piramax tablet (Pyrrolidine, altesunate) |
Safety, efficacy and safety comparative evaluation of commercially available drugs (antimalarial drugs) for mild or moderate Corona19 patients | 2 phase | 2020-05-13 |
| 4 | Chong Kun Dang Co., Ltd. | CKD-314 (Napamostat) |
Evaluation of the safety and effectiveness of commercially available drugs (anticoagulants) for hospitalized patients with COVID-19 pneumonia | 2 phase | 2020-06-17 |
| 5 | crystal Genomics Co., Ltd. |
CG-CAM20 (Duck start) |
Evaluation of the safety and effectiveness of commercially available drugs (pancreatitis drugs) for patients with COVID-19 | 2 phase | 2020-07-01 |
| 6 | Daewoong Pharmaceutical Co., Ltd. | DW1248 tablets (Duck start) |
Evaluation of the safety and effectiveness of commercially available drugs (pancreatitis drugs) for mild and moderate corona19 patients | 2/3 phase | 2020-07-06 |
| 7 | Genexine | GX-I7 | Exploring the safety and preliminary effects of clinical trial drugs (anticancer drugs) for patients with COVID-19 | 1b phase | 2020-08-07 |
| 8 | Green Cross Co., Ltd. | GC5131 | Establishment of the dose of H-Ig (high immunoglobulin) for COVID-19 patients and evaluation of efficacy and safety | 2 phase | 2020-08-20 |
| 9 | Celltrion | CT-P59 | Safety, tolerability, and virology evaluation for patients with mild corona19 | 1 phase | 2020-08-25 |
| Safety and efficacy evaluation in parallel with standard treatment for mild and moderate corona19 patients | 2/3 phase | 2020-09-17 | |||
| Preventive efficacy, virology, and safety evaluation for contact with COVID-19 patients | 3-phase | 2020-10-08 | |||
| 10 | Lily Korea | LY3009104 (Varicitinib) |
Therapeutic confirmation test of commercially available drugs (arthritis drugs) for patients with COVID-19 | 3-phase | 2020-09-07 |
| 11 | Daewoong Pharmaceutical Co., Ltd. | DWRX2003 (Niclosamide) |
Evaluation of safety, tolerability, and pharmacokinetic characteristics of commercially available drugs (parasite repellents) for healthy adults | 1 phase | 2020-10-08 |
| 12 | MSD Korea | MK-4482 | Evaluation of the safety, efficacy, and pharmacokinetics of clinical trial drugs (influenza drugs) for adult hospitalized patients with COVID-19 | 2/3 phase | 2020-10-29 |
| 13 | New Gen Therapeutics |
New Genna Pharmost Tablet (Napamostat) |
Evaluation of the safety, tolerability, and pharmacokinetic characteristics of commercially available drugs (anticoagulants) for healthy adult males | 1 phase | 2020-11-03 |
| 14 | Dong Wha Pharm. | DW2008S | Comparative evaluation of the safety and effectiveness of clinical trial drugs (asthma drugs) for moderately corona 19 patients | 2 phase | 2020-11-23 |
| 15 | Immunmed Co., Ltd. | hzVSF-v13 | The safety, efficacy, and safety of the standard therapy for clinical trial drugs (influenza drugs) for moderate and severe COVID-19 patients and hzVSF-v13 co-administration for each dose were compared with the standard therapy group alone. | 2 phase | 2020-12-07 |
※ Provided by the Ministry of Food and Drug Safety. Resale and DB ban
<저작권자(c) 연합뉴스,
Unauthorized reproduction-prohibition of redistribution>
2020/12/27 10:37 sent
–