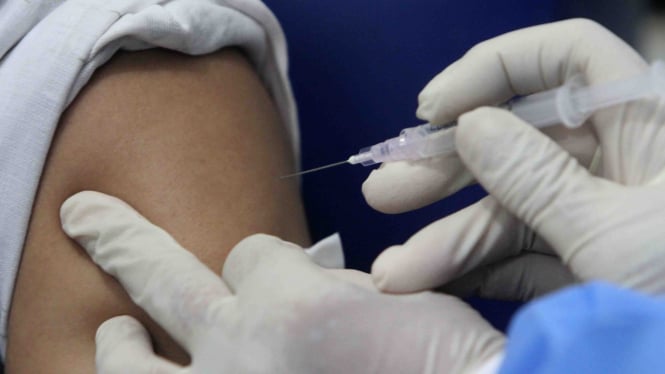
VIVA – Biotech companies Modern has started the test vaccine COVID-19 on child-12 year old child. The clinical trial for the COVID-19 vaccine will involve volunteers aged 12 to 18 years.
Trials will be held in six states in the United States including Idaho, Minnesota, New York, Oklahoma, Texas, and Utah. Federal officials have called testing vaccines in children and adolescents critical, before they are widely distributed in 2021.
Currently, Moderna and Pfizer is waiting for approval from the Food and Drug Administration, before being distributed to the public.
If given emergency approval, the first injection can be given as early as December 21, 2020 Independent.
Officials from the US Centers for Disease Control and Prevention (CDC) recommend that health workers and people living in nursing homes will be the first to receive the COVID-19 vaccine.
After health workers and the elderly in nursing homes, the CDC recommends workers to get priority in the COVID-19 vaccine injection. Workers include, among others, workers in the food and agriculture sectors, law enforcement, educators, transportation workers and emergency response workers.
So far the Pfizer vaccine has been up to 95 percent effectiveness. Meanwhile, Moderna was reported to be up to 94 percent effective. Each person should receive two doses of the vaccine.
For those who receive a shot of the Pfizer vaccine, a second dose will be given three weeks later. Meanwhile, for those who receive the Moderna vaccine, a second dose will be given four weeks later. (art)
–