Input 2021.01.06 16:25 | Revision 2021.01.06 17:05
Can’t decide to distribute, store and distribute to hospitals nationwide
Logistics industry struggles to distribute vaccines due to lack of money and high risk
Government to prepare specific vaccination plan soon–
–
Moreover, in the case of Modena (May) and Pfizer (undecided) vaccines that show efficacy only when transported and stored at -20 to 70°C, distribution in Korea is expected to be more difficult. If a proper cold chain (low temperature distribution system) is not in place, there is a possibility that the hard-earned vaccine will be discarded. Moreover, there is only one cryogenic warehouse in Korea where vaccines can be stored. The logistics industry is not willing to come to the point that vaccine distribution is difficult.
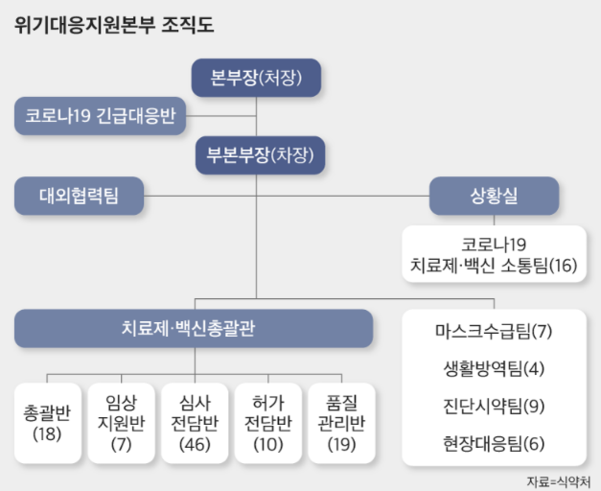
According to the Ministry of Food and Drug Safety on the 6th, the department in charge of distribution and transportation of Corona 19 vaccines in Korea is the quality control group under the Corona Treatment and Vaccine General Office of the’Corona 19 Crisis Response Support Headquarters’, a task force (TF) launched on the 31st of last month. .
When the Korean government makes a vaccine supply contract with a pharmaceutical company, the quality control group is responsible for managing and supervising the compliance of the cold chain standard, the manufacturer’s shipment process, and the domestic distribution process when importing. It also prepares guidelines for guidelines in the vaccine distribution process.
–
–
In this regard, an official from the Ministry of Food and Drug Safety said, “The vaccine contract was signed and the introduction was also ready, but we know that there are criticisms that securing distribution and distribution warehouses has been delayed.” ) You have to figure out the quantity, but all of these are not fixed.” The official said, “We are working hard to get the AstraZeneca vaccine licensed in February, even one day ahead,” and said, “There are variables, but we are going through the procedure with the goal of obtaining a vaccine approval in February. (Vaccination) will not pass into March. “I said.
The AstraZeneca vaccine is scheduled to be produced by consignment (CMO) at SK Bioscience in Songdo, Incheon, but it does not digest all of the domestic supply. It is also made in the United States and the United Kingdom and is expected to enter Korea. Therefore, the selection of logistics companies in charge of domestic distribution and storage emerged as a major task.
Currently, Yongma Logis and Ilyang Palm Logis are prominently mentioned as domestic Corona 19 vaccine distribution companies. Both companies have the know-how to deliver medicines professionally. It is known that AstraZeneca vaccine, which requires low temperature distribution at 3~8℃, can respond sufficiently.
However, just as there was a room temperature exposure accident last year during the distribution of influenza (influenza), the risk of distribution of the Corona 19 vaccine is considerable. Because of this, large logistics companies are not willing to distribute the Corona 19 vaccine. In particular, Modena (minus 20℃) and Pfizer (minus 70℃) vaccines are effective only when stored and distributed at cryogenic temperatures, but the industry judges that the domestic logistics distribution and storage infrastructure is not sufficient yet.
An official at Ilyang Farm Logis said, “It is true that discussions about distribution with the government have come and gone at the level of the Ilyang Group. Unlike regular delivery, the case of the Corona 19 vaccine requires special conditions for ultra-low temperature distribution.” The official said, “We have been building drug delivery and distribution for a long time, but it has been built on a small scale,” he said. “In case of large quantities, investments in the group level, such as the introduction of special vehicles, should be reviewed, but there was discussion recently, but nothing has been decided yet. “He said.
–

–
An official in the logistics industry said, “Even if you understand the specificity of the Corona 19 vaccine, there is no reason to take a risk if there is nothing left as a vaccine distribution,” said “the rumor has (Korea’s cryogenic side) 264.4㎡ (80 pyeong) one-year contract. It is said that 5 billion won was offered to the company, but it was 5 million won per 3.3㎡.
In the case of Samsung SDS, which is known to have participated in vaccine distribution simulations with Yongmar Logis and Korea Cryogenics, it is negative about direct vaccine distribution. A company official said, “The simulation was only to test whether the IT logistics management system (cello) possessed by Samsung SDS was suitable for vaccine distribution,” he said. “There was no decision regarding direct distribution.” It was found that CJ Logistics and Hyundai Glovis do not currently have a clear position regarding vaccine distribution.
Some point out that the government believes in the existing flu vaccine supply chain and is looking at the distribution of the Corona 19 vaccine easily. In order to prevent the spread of the Corona 19 vaccine, it must be done quickly after the decision to inoculate, but it is against common sense that the logistics and distribution network have not been prepared in advance because the amount of introduction or the timing of inoculation has not been determined.
–

–
Meanwhile, the quarantine authorities plan to launch the’Corona 19 Vaccination Response Promotion Team’ under the Central Defense Response Headquarters (Disease Management Office) on the 8th. In addition, it was decided to operate the Vaccination Specialist Committee to prepare detailed vaccination plans such as the corona19 vaccination target and period, vaccination interval, and adverse reaction management system. A specific plan will be released within this month.
–
– .
![[단독] One month left for the corona vaccination… The government has not even decided how to distribute it nationwide [단독] One month left for the corona vaccination… The government has not even decided how to distribute it nationwide](https://image.chosun.com/sitedata/image/202101/06/2021010602425_0.jpg)

